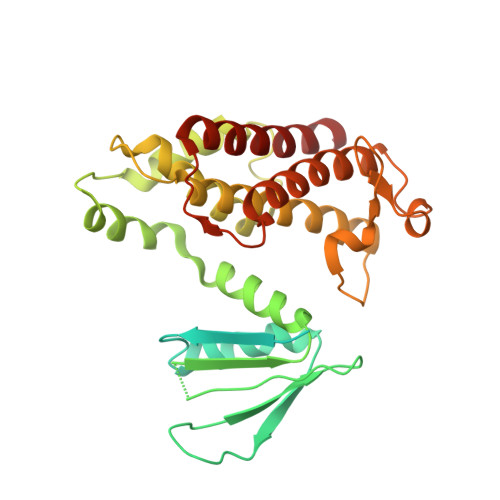
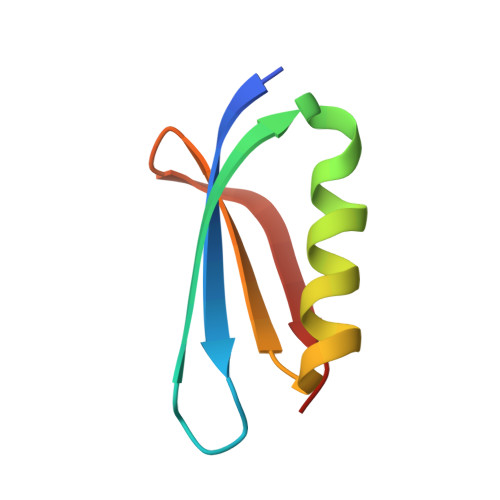
Structural insights into the inhibition mechanism of bacterial toxin LsoA by bacteriophage antitoxin Dmd
Wan, H., Otsuka, Y., Gao, Z.Q., Wei, Y., Chen, Z., Masuda, M., Yonesaki, T., Zhang, H., Dong, Y.H.(2016) Mol Microbiol 101: 757-769
- PubMed: 27169810
- DOI: https://doi.org/10.1111/mmi.13420
- Primary Citation of Related Structures:
5HY3 - PubMed Abstract:
Bacteria have obtained a variety of resistance mechanisms including toxin-antitoxin (TA) systems against bacteriophages (phages), whereas phages have also evolved to overcome bacterial anti-phage mechanisms. Dmd from T4 phage can suppress the toxicities of homologous toxins LsoA and RnlA from Escherichia coli, representing the first example of a phage antitoxin against multiple bacterial toxins in known TA systems. Here, the crystal structure of LsoA-Dmd complex showed Dmd is inserted into the deep groove between the N-terminal repeated domain (NRD) and the Dmd-binding domain (DBD) of LsoA. The NRD shifts significantly from a 'closed' to an 'open' conformation upon Dmd binding. Site-directed mutagenesis of Dmd revealed the conserved residues (W31 and N40) are necessary for LsoA binding and the toxicity suppression as determined by pull-down and cell toxicity assays. Further mutagenesis identified the conserved Dmd-binding residues (R243, E246 and R305) of LsoA are vital for its toxicity, and suggested Dmd and LsoB may possess different inhibitory mechanisms against LsoA toxicity. Our structure-function studies demonstrate Dmd can recognize LsoA and inhibit its toxicity by occupying the active site possibly via substrate mimicry. These findings have provided unique insights into the defense and counter-defense mechanisms between bacteria and phages in their co-evolution.
Organizational Affiliation:
Beijing Synchrotron Radiation Facility, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing, 100049, China.