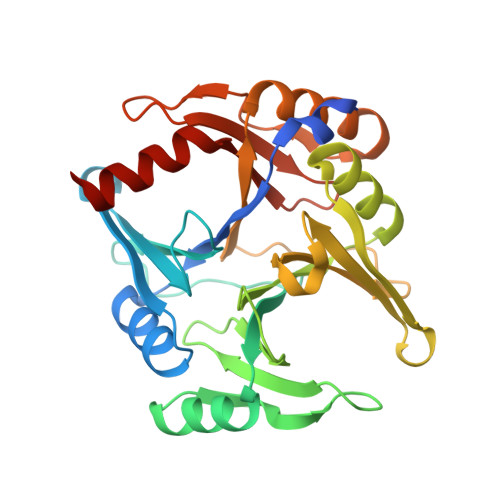
Structure of the novel monomeric glyoxalase I from Zea mays.
Turra, G.L., Agostini, R.B., Fauguel, C.M., Presello, D.A., Andreo, C.S., Gonzalez, J.M., Campos-Bermudez, V.A.(2015) Acta Crystallogr D Biol Crystallogr 71: 2009-2020
- PubMed: 26457425
- DOI: https://doi.org/10.1107/S1399004715015205
- Primary Citation of Related Structures:
5D7Z - PubMed Abstract:
The glyoxalase system is ubiquitous among all forms of life owing to its central role in relieving the cell from the accumulation of methylglyoxal, a toxic metabolic byproduct. In higher plants, this system is upregulated under diverse metabolic stress conditions, such as in the defence response to infection by pathogenic microorganisms. Despite their proven fundamental role in metabolic stresses, plant glyoxalases have been poorly studied. In this work, glyoxalase I from Zea mays has been characterized both biochemically and structurally, thus reporting the first atomic model of a glyoxalase I available from plants. The results indicate that this enzyme comprises a single polypeptide with two structurally similar domains, giving rise to two lateral concavities, one of which harbours a functional nickel(II)-binding active site. The putative function of the remaining cryptic active site remains to be determined.
Organizational Affiliation:
Centro de Estudios Fotosintéticos y Bioquímicos (CEFOBI-CONICET), Universidad Nacional de Rosario, Suipacha 531, S2002LRK Rosario, Argentina.