Structural Basis for Heme Recognition by HmuT Responsible for Heme Transport to the Heme Transporter in Corynebacterium glutamicum
Muraki, N., Aono, S.(2015) Chem Lett
Experimental Data Snapshot
(2015) Chem Lett
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ABC-type transporter, periplasmic component | 344 | Corynebacterium glutamicum ATCC 13032 | Mutation(s): 0 Gene Names: Cgl0389 | 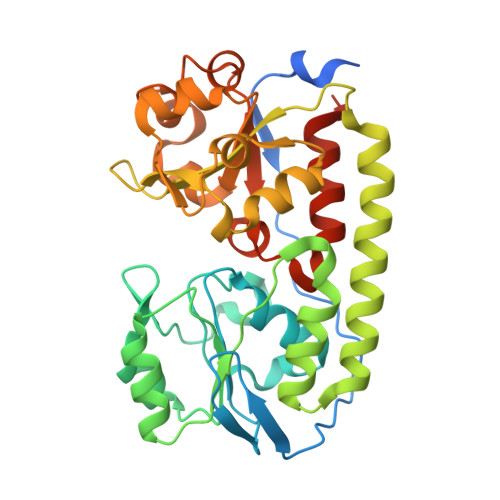 | |
UniProt | |||||
Find proteins for Q8NTB8 (Corynebacterium glutamicum (strain ATCC 13032 / DSM 20300 / BCRC 11384 / JCM 1318 / LMG 3730 / NCIMB 10025)) Explore Q8NTB8 Go to UniProtKB: Q8NTB8 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8NTB8 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| HEM Query on HEM | B [auth A] | PROTOPORPHYRIN IX CONTAINING FE C34 H32 Fe N4 O4 KABFMIBPWCXCRK-RGGAHWMASA-L |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 73.04 | α = 90 |
| b = 73.04 | β = 90 |
| c = 147.32 | γ = 90 |
| Software Name | Purpose |
|---|---|
| iMOSFLM | data processing |
| SCALA | data scaling |
| PHASER | phasing |
| Coot | model building |
| PHENIX | refinement |