The Crystal Structure of the Gst-Like Domains Complex of Eprs C92Sc105Sc123S Mutant-Aimp2
Cho, H.Y., Kang, B.S.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| BIFUNCTIONAL GLUTAMATE/PROLINE--TRNA LIGASE | 175 | Homo sapiens | Mutation(s): 3 EC: 6.1.1.15 (PDB Primary Data), 6.1.1.17 (PDB Primary Data) | 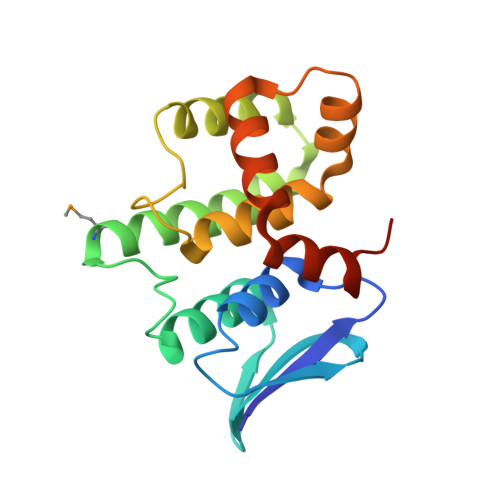 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P07814 (Homo sapiens) Explore P07814 Go to UniProtKB: P07814 | |||||
PHAROS: P07814 GTEx: ENSG00000136628 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P07814 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| AMINOACYL TRNA SYNTHASE COMPLEX-INTERACTING MULTIFUNCTIONA L PROTEIN 2 | 240 | Homo sapiens | Mutation(s): 0 | 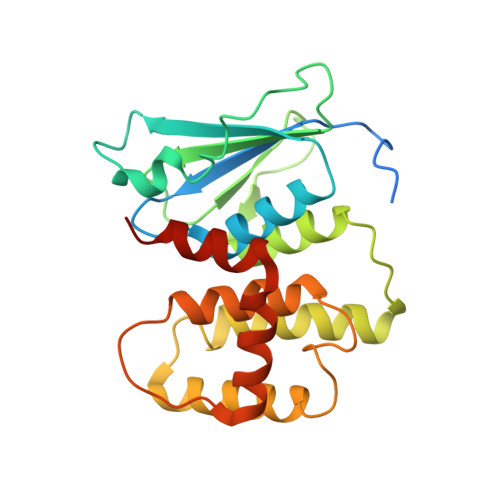 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q13155 (Homo sapiens) Explore Q13155 Go to UniProtKB: Q13155 | |||||
PHAROS: Q13155 GTEx: ENSG00000106305 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q13155 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A, C, E, G | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 94.687 | α = 90 |
| b = 112.793 | β = 90 |
| c = 181.249 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHENIX | phasing |