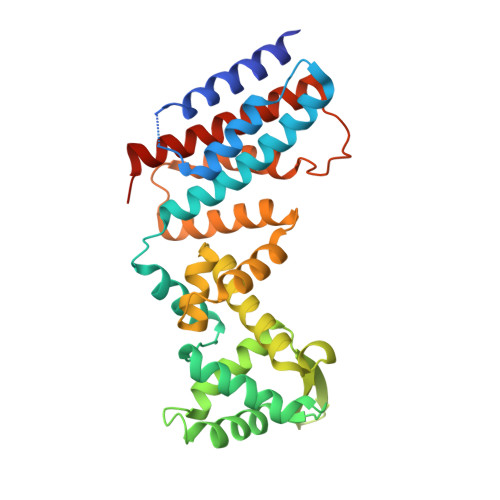
New Insights into the RNA-Binding and E3 Ubiquitin Ligase Activities of Roquins.
Zhang, Q., Fan, L., Hou, F., Dong, A., Wang, Y.X., Tong, Y.(2015) Sci Rep 5: 15660-15660
- PubMed: 26489670
- DOI: https://doi.org/10.1038/srep15660
- Primary Citation of Related Structures:
4Z30, 4Z31 - PubMed Abstract:
Roquins are a family of highly conserved RNA-binding proteins that also contain a RING-type E3 ubiquitin ligase domain. They repress constitutive decay elements containing mRNAs and play a critical role in RNA homeostasis and immunological self-tolerance. Here we present the crystal structures of the RNA-binding region of Roquin paralog RC3H2 in both apo- and RNA-bound forms. The RNA-binding region has a bipartite architecture composed of ROQ and HEPN domains, and can bind to stem-loop and double-stranded RNAs simultaneously. The two domains undergo a large orientation change to accommodate RNA duplex binding. We profiled E2 ubiquitin-conjugating enzymes that pair with Roquins and found that RC3H1 and RC3H2 interact with two sets of overlapping but not identical E2 enzymes to drive the assembly of polyubiquitin chains of different linkages. Crystal structures, small-angle X-ray scattering, and E2 profiling revealed that while the two paralogs are highly homologous, RC3H2 and RC3H1 are different in their structures and functions. We also demonstrated that RNA duplex binding to RC3H2 cross-talks with its E3 ubiquitin ligase function using an in vitro auto-ubiquitination assay.
Organizational Affiliation:
Structural Genomics Consortium, University of Toronto, Toronto, ON M5G 1L7, Canada.