Structural Characterization of Substrate and Inhibitor Binding to Farnesyl Pyrophosphate Synthase from Pseudomonas Aeruginosa.
Schmidberger, J.W., Schnell, R., Schneider, G.(2015) Acta Crystallogr D Biol Crystallogr 71: 721
- PubMed: 25760619
- DOI: https://doi.org/10.1107/S1399004715001121
- Primary Citation of Related Structures:
3ZCD, 3ZL6, 3ZMB, 3ZMC, 3ZOU, 4UMJ - PubMed Abstract:
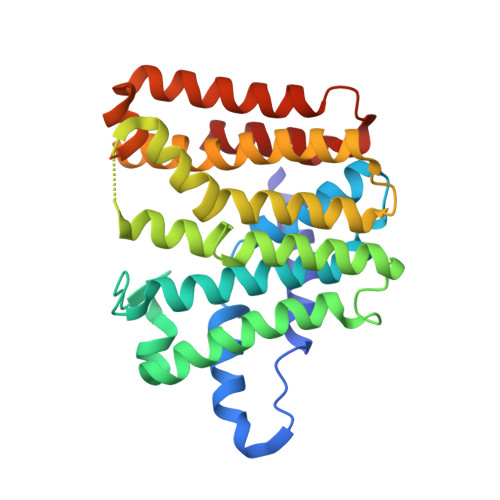
Locus PA4043 in the genome of Pseudomonas aeruginosa PAO1 has been annotated as coding for a farnesyl pyrophosphate synthase (FPPS). This open reading frame was cloned and expressed recombinantly in Escherichia coli. The dimeric enzyme shows farnesyl pyrophosphate synthase activity and is strongly inhibited by ibandronate and zoledronate, drugs that are presently in clinical use. The structures of the unliganded enzyme and complexes with the substrate geranyl diphosphate (GPP), the inhibitor ibandronate and two compounds obtained from a differential scanning fluorimetry-based screen of a fragment library were determined by X-ray crystallography to resolutions of better than 2.0 Å. The enzyme shows the typical α-helical fold of farnesyl pyrophosphate synthases. The substrate GPP binds in the S1 substrate site in an open conformation of the enzyme. In the enzyme-ibandronate complex three inhibitor molecules are bound in the active site of the enzyme. One inhibitor molecule occupies the allylic substrate site (S1) of each subunit, as observed in complexes of nitrogen-containing bisphosphonate inhibitors of farnesyl synthases from other species. Two (in subunit A) and one (in subunit B) additional ibandronate molecules are bound in the active site. The structures of the fragment complexes show two molecules bound in a hydrophobic pocket adjacent to the active site. This allosteric pocket, which has previously only been described for FPPS from eukaryotic organisms, is thus also present in enzymes from pathogenic prokaryotes and might be utilized for the design of inhibitors of bacterial FPPS with a different chemical scaffold to the highly charged bisphosphonates, which are less likely to pass bacterial membranes.
Organizational Affiliation:
Department of Medical Biochemistry and Biophysics, Karolinska Institutet, SE-171 77 Stockholm, Sweden.