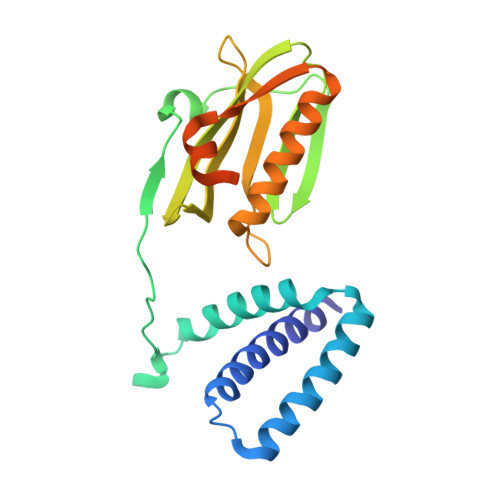
The Influenza A Virus Protein NS1 Displays Structural Polymorphism.
Carrillo, B., Choi, J.M., Bornholdt, Z.A., Sankaran, B., Rice, A.P., Prasad, B.V.(2014) J Virol 88: 4113-4122
- PubMed: 24478439
- DOI: https://doi.org/10.1128/JVI.03692-13
- Primary Citation of Related Structures:
4OPA, 4OPH - PubMed Abstract:
NS1 of influenza A virus is a potent antagonist of host antiviral interferon responses. This multifunctional protein with two distinctive domains, an RNA-binding domain (RBD) and an effector domain (ED) separated by a linker region (LR), is implicated in replication, pathogenesis, and host range. Although the structures of individual domains of NS1 from different strains of influenza viruses have been reported, the only structure of full-length NS1 available to date is from an H5N1 strain (A/Vietnam/1203/2004). By carrying out crystallographic analyses of full-length H6N6-NS1 (A/blue-winged teal/MN/993/1980) and an LR deletion mutant, combined with mutational analysis, we show here that these full-length NS1 structures provide an exquisite structural sampling of various conformational states of NS1 that based on the orientation of the ED with respect to RBD can be summarized as "open," "semi-open," and "closed" conformations. Our studies show that preference for these states is clearly dictated by determinants such as linker length, residue composition at position 71, and a mechanical hinge, providing a structural basis for strain-dependent functional variations in NS1. Because of the flexibility inherent in the LR, any particular NS1 could sample the conformational space around these states to engage ED in different quaternary interactions so that it may participate in specific protein-protein or protein-RNA interactions to allow for the known multifunctionality of NS1. We propose that such conformational plasticity provides a mechanism for autoregulating NS1 functions, depending on its temporal distribution, posttranslational modifications, and nuclear or cellular localization, during the course of virus infection.
Organizational Affiliation:
Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas, USA.