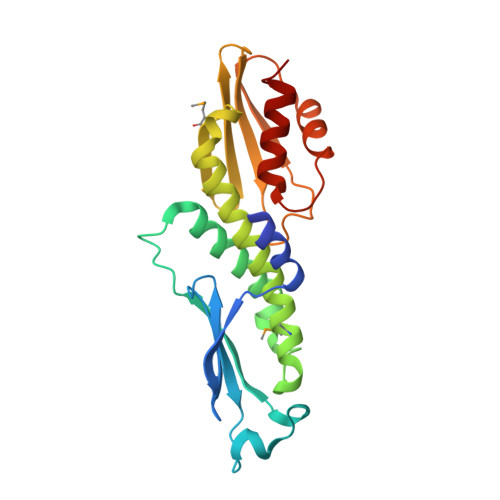
Modular Assembly of RWD Domains on the Mis12 Complex Underlies Outer Kinetochore Organization.
Petrovic, A., Mosalaganti, S., Keller, J., Mattiuzzo, M., Overlack, K., Krenn, V., De Antoni, A., Wohlgemuth, S., Cecatiello, V., Pasqualato, S., Raunser, S., Musacchio, A.(2014) Mol Cell 53: 591-605
- PubMed: 24530301
- DOI: https://doi.org/10.1016/j.molcel.2014.01.019
- Primary Citation of Related Structures:
4NF9, 4NFA - PubMed Abstract:
Faithful chromosome segregation is mandatory for cell and organismal viability. Kinetochores, large protein assemblies embedded in centromeric chromatin, establish a mechanical link between chromosomes and spindle microtubules. The KMN network, a conserved 10-subunit kinetochore complex, harbors the microtubule-binding interface. RWD domains in the KMN subunits Spc24 and Spc25 mediate kinetochore targeting of the microtubule-binding subunits by interacting with the Mis12 complex, a KMN subcomplex that tethers directly onto the underlying chromatin layer. Here, we show that Knl1, a KMN subunit involved in mitotic checkpoint signaling, also contains RWD domains that bind the Mis12 complex and that mediate kinetochore targeting of Knl1. By reporting the first 3D electron microscopy structure of the KMN network, we provide a comprehensive framework to interpret how interactions of RWD-containing proteins with the Mis12 complex shape KMN network topology. Our observations unveil a regular pattern in the construction of the outer kinetochore.
Organizational Affiliation:
Department of Mechanistic Cell Biology, Max Planck Institute of Molecular Physiology, Otto-Hahn-Straße 11, 44227 Dortmund, Germany.