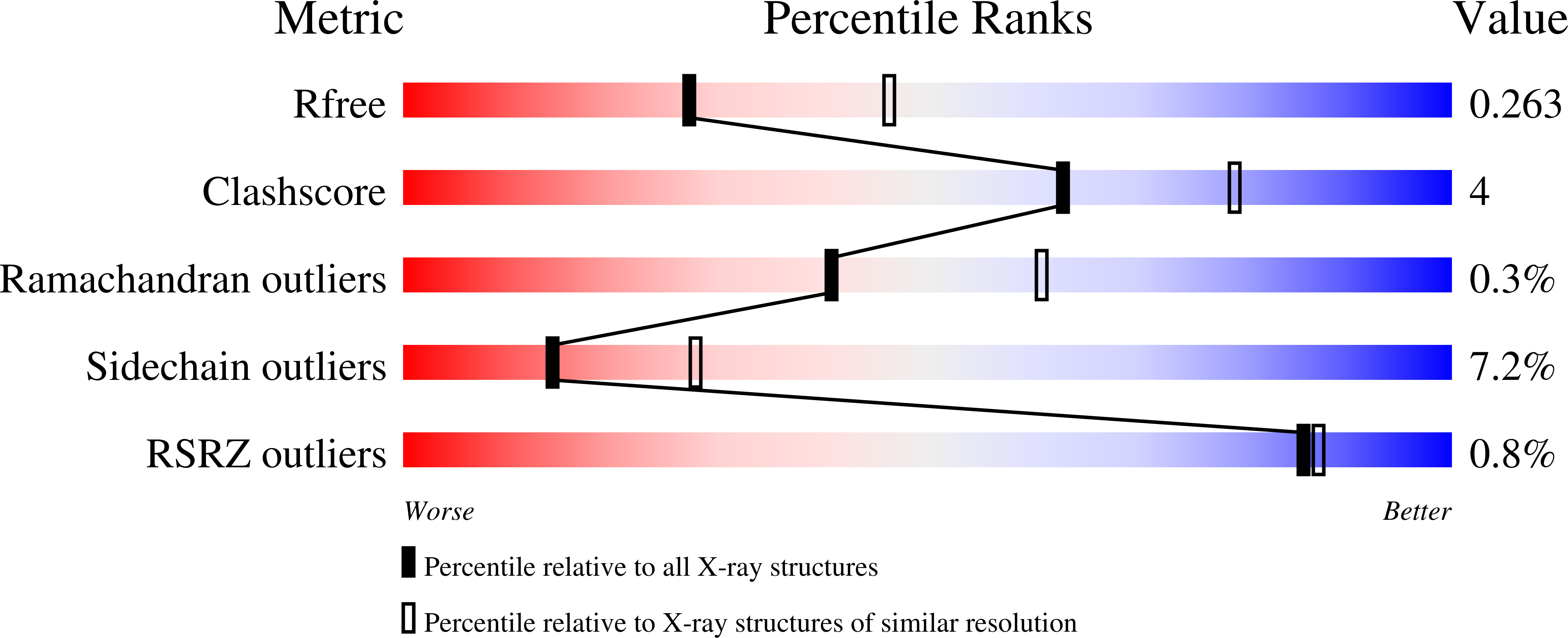
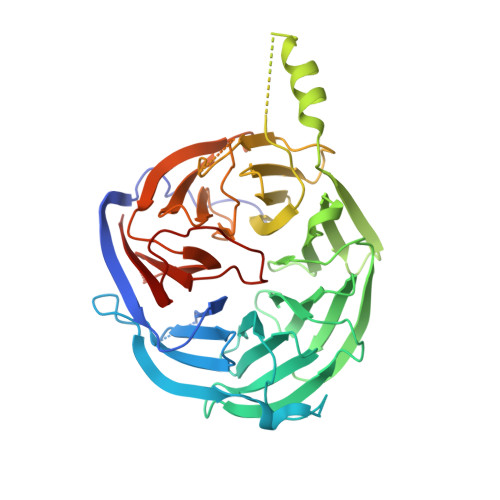
Structural and functional analysis of the U3 snoRNA binding protein Rrp9.
Zhang, L., Lin, J., Ye, K.(2013) RNA 19: 701-711
- PubMed: 23509373
- DOI: https://doi.org/10.1261/rna.037580.112
- Primary Citation of Related Structures:
4J0W, 4J0X - PubMed Abstract:
The U3 snoRNA is required for 18S rRNA processing and small subunit ribosome formation in eukaryotes. Different from other box C/D snoRNAs, U3 contains an extra 5' domain that pairs with pre-rRNA and a unique B/C motif essential for recruitment of the U3-specific Rrp9 protein. Here, we analyze the structure and function of Rrp9 with crystallographic, biochemical, and cellular approaches. Rrp9 is composed of a WD repeat domain and an N-terminal region. The crystal structures of the WD domain of yeast Rrp9 and its human ortholog U3-55K were determined, revealing a typical seven-bladed propeller fold. Several conserved surface patches on the WD domain were identified, and their function in RNP assembly and yeast growth were analyzed by mutagenesis. Prior association of Snu13 with the B/C motif was found to enhance the specific binding of the WD domain. We show that a conserved 7bc loop is crucial for specific recognition of U3, nucleolar localization of Rrp9, and yeast growth. In addition, we show that the N-terminal region of Rrp9 contains a bipartite nuclear localization signal that is dispensable for nucleolar localization. Our results provide insight into the functional sites of Rrp9.
Organizational Affiliation:
Graduate School of Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China.