Crystal Structure of Putative Thioredoxin Reductase TrxB from Bacillus anthracis
Maltseva, N., Kim, Y., Kwon, K., Anderson, W.F., Joachimiak, A., Center for Structural Genomics of Infectious Diseases (CSGID)To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Putative thioredoxin reductase | 304 | Bacillus anthracis str. 'Ames Ancestor | Mutation(s): 0 Gene Names: BA_2768, GBAA_2768 | 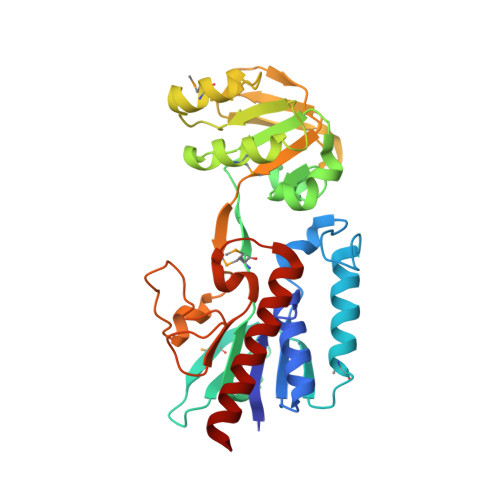 | |
UniProt | |||||
Find proteins for A0A6L8PLA6 (Bacillus anthracis) Explore A0A6L8PLA6 Go to UniProtKB: A0A6L8PLA6 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A6L8PLA6 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Putative thioredoxin reductase | 304 | Bacillus anthracis str. 'Ames Ancestor | Mutation(s): 0 Gene Names: BA_2768, GBAA_2768 |  | |
UniProt | |||||
Find proteins for A0A6L8PLA6 (Bacillus anthracis) Explore A0A6L8PLA6 Go to UniProtKB: A0A6L8PLA6 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A6L8PLA6 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| FAD Query on FAD | E [auth A], G [auth B], H [auth C], K [auth D] | FLAVIN-ADENINE DINUCLEOTIDE C27 H33 N9 O15 P2 VWWQXMAJTJZDQX-UYBVJOGSSA-N |  | ||
| GOL Query on GOL | M [auth D] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| MG Query on MG | F [auth A], I [auth C], J [auth C], L [auth D] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Modified Residues 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A, B, D | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| MSO Query on MSO | C | L-PEPTIDE LINKING | C5 H11 N O3 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 82.558 | α = 90 |
| b = 54.096 | β = 93.04 |
| c = 140.037 | γ = 90 |
| Software Name | Purpose |
|---|---|
| SBC-Collect | data collection |
| HKL-3000 | data collection |
| HKL-3000 | phasing |
| MOLREP | phasing |
| PHENIX | refinement |
| HKL-3000 | data reduction |
| HKL-3000 | data scaling |