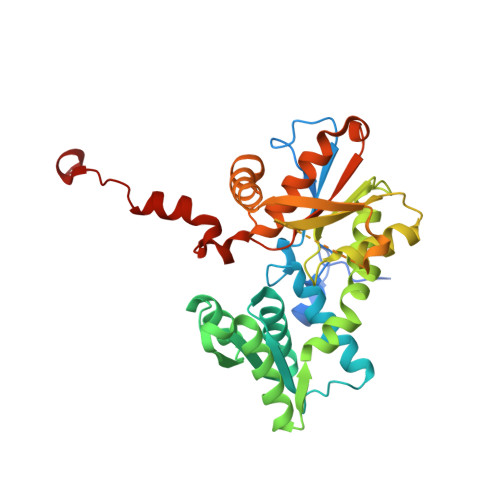
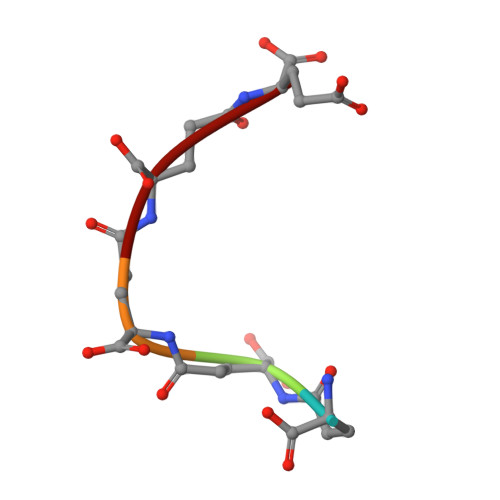
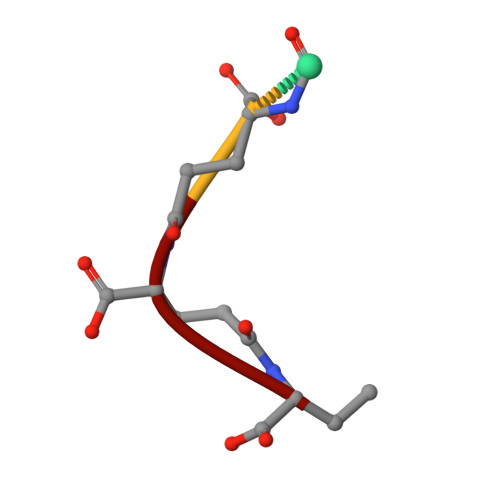
Structure of Leishmania Major Cysteine Synthase.
Fyfe, P.K., Westrop, G.D., Ramos, T., Muller, S., Coombs, G.H., Hunter, W.N.(2012) Acta Crystallogr Sect F Struct Biol Cryst Commun 68: 738
- PubMed: 22750854
- DOI: https://doi.org/10.1107/S1744309112019124
- Primary Citation of Related Structures:
4AIR - PubMed Abstract:
Cysteine biosynthesis is a potential target for drug development against parasitic Leishmania species; these protozoa are responsible for a range of serious diseases. To improve understanding of this aspect of Leishmania biology, a crystallographic and biochemical study of L. major cysteine synthase has been undertaken, seeking to understand its structure, enzyme activity and modes of inhibition. Active enzyme was purified, assayed and crystallized in an orthorhombic form with a dimer in the asymmetric unit. Diffraction data extending to 1.8 Å resolution were measured and the structure was solved by molecular replacement. A fragment of γ-poly-D-glutamic acid, a constituent of the crystallization mixture, was bound in the enzyme active site. Although a D-glutamate tetrapeptide had insignificant inhibitory activity, the enzyme was competitively inhibited (K(i) = 4 µM) by DYVI, a peptide based on the C-terminus of the partner serine acetyltransferase with which the enzyme forms a complex. The structure surprisingly revealed that the cofactor pyridoxal phosphate had been lost during crystallization.
Organizational Affiliation:
Division of Biological Chemistry and Drug Discovery, College of Life Sciences, University of Dundee, Dundee DD1 5EH, Scotland.