Crystal structure, mutational analysis and RNA-dependent ATPase activity of the yeast DEAD-box pre-mRNA splicing factor Prp28.
Jacewicz, A., Schwer, B., Smith, P., Shuman, S.(2014) Nucleic Acids Res 42: 12885-12898
- PubMed: 25303995
- DOI: https://doi.org/10.1093/nar/gku930
- Primary Citation of Related Structures:
4W7S - PubMed Abstract:
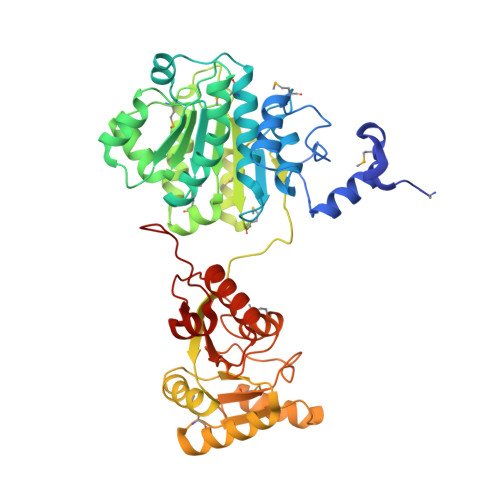
Yeast Prp28 is a DEAD-box pre-mRNA splicing factor implicated in displacing U1 snRNP from the 5' splice site. Here we report that the 588-aa Prp28 protein consists of a trypsin-sensitive 126-aa N-terminal segment (of which aa 1-89 are dispensable for Prp28 function in vivo) fused to a trypsin-resistant C-terminal catalytic domain. Purified recombinant Prp28 and Prp28-(127-588) have an intrinsic RNA-dependent ATPase activity, albeit with a low turnover number. The crystal structure of Prp28-(127-588) comprises two RecA-like domains splayed widely apart. AMPPNP•Mg2+ is engaged by the proximal domain, with proper and specific contacts from Phe194 and Gln201 (Q motif) to the adenine nucleobase. The triphosphate moiety of AMPPNP•Mg2+ is not poised for catalysis in the open domain conformation. Guided by the Prp28•AMPPNP structure, and that of the Drosophila Vasa•AMPPNP•Mg2+•RNA complex, we targeted 20 positions in Prp28 for alanine scanning. ATP-site components Asp341 and Glu342 (motif II) and Arg527 and Arg530 (motif VI) and RNA-site constituent Arg476 (motif Va) are essential for Prp28 activity in vivo. Synthetic lethality of double-alanine mutations highlighted functionally redundant contacts in the ATP-binding (Phe194-Gln201, Gln201-Asp502) and RNA-binding (Arg264-Arg320) sites. Overexpression of defective ATP-site mutants, but not defective RNA-site mutants, elicited severe dominant-negative growth defects.
Organizational Affiliation:
Molecular Biology Program, Sloan-Kettering Institute, New York, NY 10065, USA.