Limited proteolysis improves E.coli Hfq crystal structure resolution
Feng, S.Q., Si, Y.L., Song, C.Y., Wang, P.Q., Su, J.Y.(2015) Zhongguo Sheng Wu Hua Xue Yu Fen Zi Sheng Wu Xue Bao 9: 845-851
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
(2015) Zhongguo Sheng Wu Hua Xue Yu Fen Zi Sheng Wu Xue Bao 9: 845-851
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| RNA-binding protein Hfq | 67 | Escherichia coli K-12 | Mutation(s): 0 | 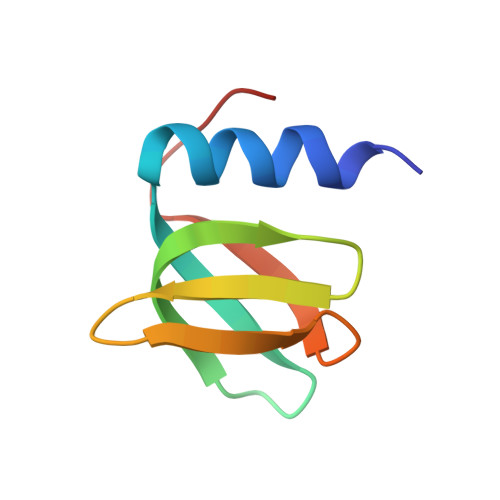 | |
UniProt | |||||
Find proteins for P0A6X3 (Escherichia coli (strain K12)) Explore P0A6X3 Go to UniProtKB: P0A6X3 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0A6X3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 61.32 | α = 90 |
| b = 61.32 | β = 90 |
| c = 28.09 | γ = 120 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |