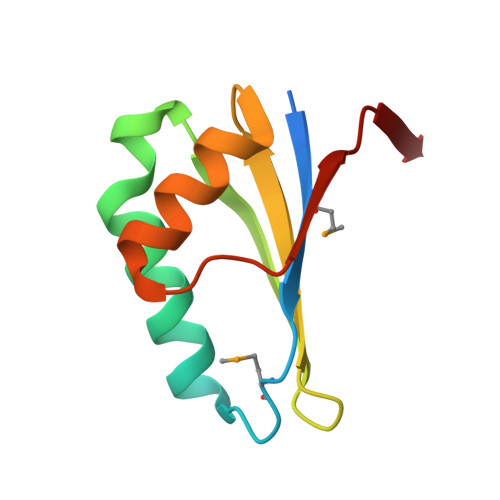
Streptomyces coelicolor SCO4226 Is a Nickel Binding Protein.
Lu, M., Jiang, Y.L., Wang, S., Jin, H., Zhang, R.G., Virolle, M.J., Chen, Y., Zhou, C.Z.(2014) PLoS One 9: e109660-e109660
- PubMed: 25285530
- DOI: https://doi.org/10.1371/journal.pone.0109660
- Primary Citation of Related Structures:
4OI3, 4OI6 - PubMed Abstract:
The open reading frame SCO4226 of Streptomyces coelicolor A3(2) encodes an 82-residue hypothetical protein. Biochemical assays revealed that each SCO4226 dimer binds four nickel ions. To decipher the molecular function, we solved the crystal structures of SCO4226 in both apo- and nickel-bound (Ni-SCO4226) forms at 1.30 and 2.04 Å resolution, respectively. Each subunit of SCO4226 dimer adopts a canonical ferredoxin-like fold with five β-strands flanked by two α-helices. In the structure of Ni-SCO4226, four nickel ions are coordinated at the surface of the dimer. Further biochemical assays suggested that the binding of Ni2+ triggers the self-aggregation of SCO4226 in vitro. In addition, RT-qPCR assays demonstrated that the expression of SCO4226 gene in S. coelicolor is specifically up-regulated by the addition of Ni2+, but not other divalent ions such as Cu2+, Mn2+ or Co2+. All these results suggested that SCO4226 acts as a nickel binding protein, probably required for nickel sequestration and/or detoxification.
Organizational Affiliation:
Hefei National Laboratory for Physical Sciences at the Microscale and School of Life Sciences, University of Science and Technology of China, Hefei, Anhui, People's Republic of China; Department of Biology, South University of Sciences and Technology of China, Shenzhen, Guangdong, People's Republic of China.