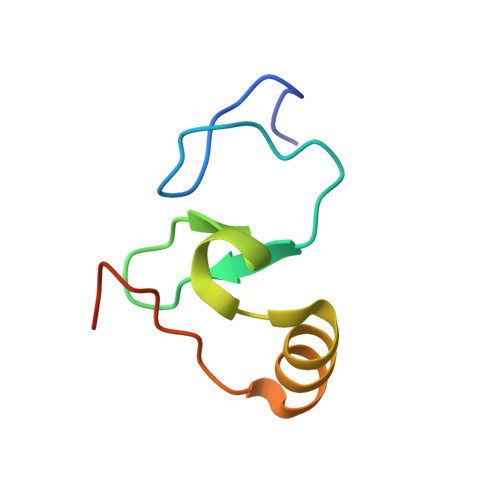
Dido3 PHD Modulates Cell Differentiation and Division.
Gatchalian, J., Futterer, A., Rothbart, S.B., Tong, Q., Rincon-Arano, H., Sanchez de Diego, A., Groudine, M., Strahl, B.D., Martinez-A, C., van Wely, K.H., Kutateladze, T.G.(2013) Cell Rep 4: 148-158
- PubMed: 23831028
- DOI: https://doi.org/10.1016/j.celrep.2013.06.014
- Primary Citation of Related Structures:
4L7X - PubMed Abstract:
Death Inducer Obliterator 3 (Dido3) is implicated in the maintenance of stem cell genomic stability and tumorigenesis. Here, we show that Dido3 regulates the expression of stemness genes in embryonic stem cells through its plant homeodomain (PHD) finger. Binding of Dido3 PHD to histone H3K4me3 is disrupted by threonine phosphorylation that triggers Dido3 translocation from chromatin to the mitotic spindle. The crystal structure of Dido3 PHD in complex with H3K4me3 reveals an atypical aromatic-cage-like binding site that contains a histidine residue. Biochemical, structural, and mutational analyses of the binding mechanism identified the determinants of specificity and affinity and explained the inability of homologous PHF3 to bind H3K4me3. Together, our findings reveal a link between the transcriptional control in embryonic development and regulation of cell division.
Organizational Affiliation:
Department of Pharmacology, University of Colorado School of Medicine, Aurora, CO 80045, USA.