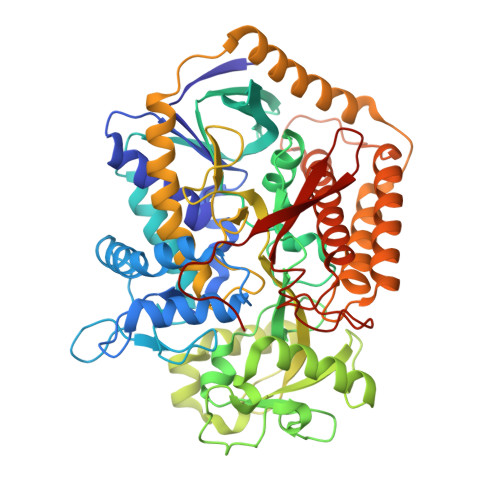
Plasticity of the Quinone-binding Site of the Complex II Homolog Quinol:Fumarate Reductase.
Singh, P.K., Sarwar, M., Maklashina, E., Kotlyar, V., Rajagukguk, S., Tomasiak, T.M., Cecchini, G., Iverson, T.M.(2013) J Biol Chem 288: 24293-24301
- PubMed: 23836905
- DOI: https://doi.org/10.1074/jbc.M113.487082
- Primary Citation of Related Structures:
4KX6 - PubMed Abstract:
Respiratory processes often use quinone oxidoreduction to generate a transmembrane proton gradient, making the 2H(+)/2e(-) quinone chemistry important for ATP synthesis. There are a variety of quinones used as electron carriers between bioenergetic proteins, and some respiratory proteins can functionally interact with more than one quinone type. In the case of complex II homologs, which couple quinone chemistry to the interconversion of succinate and fumarate, the redox potentials of the biologically available ubiquinone and menaquinone aid in driving the chemical reaction in one direction. In the complex II homolog quinol:fumarate reductase, it has been demonstrated that menaquinol oxidation requires at least one proton shuttle, but many of the remaining mechanistic details of menaquinol oxidation are not fully understood, and little is known about ubiquinone reduction. In the current study, structural and computational studies suggest that the sequential removal of the two menaquinol protons may be accompanied by a rotation of the naphthoquinone ring to optimize the interaction with a second proton shuttling pathway. However, kinetic measurements of site-specific mutations of quinol:fumarate reductase variants show that ubiquinone reduction does not use the same pathway. Computational docking of ubiquinone followed by mutagenesis instead suggested redundant proton shuttles lining the ubiquinone-binding site or from direct transfer from solvent. These data show that the quinone-binding site provides an environment that allows multiple amino acid residues to participate in quinone oxidoreduction. This suggests that the quinone-binding site in complex II is inherently plastic and can robustly interact with different types of quinones.
Organizational Affiliation:
Department of Pharmacology, Vanderbilt University Medical Center, Nashville, Tennessee 37232, USA.