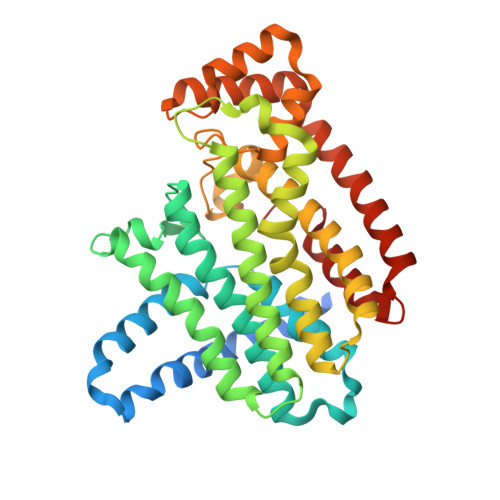
Ternary complex structures of human farnesyl pyrophosphate synthase bound with a novel inhibitor and secondary ligands provide insights into the molecular details of the enzyme's active site closure.
Park, J., Lin, Y.S., De Schutter, J.W., Tsantrizos, Y.S., Berghuis, A.M.(2012) BMC Struct Biol 12: 32-32
- PubMed: 23234314
- DOI: https://doi.org/10.1186/1472-6807-12-32
- Primary Citation of Related Structures:
4H5C, 4H5D, 4H5E - PubMed Abstract:
Human farnesyl pyrophosphate synthase (FPPS) controls intracellular levels of farnesyl pyrophosphate, which is essential for various biological processes. Bisphosphonate inhibitors of human FPPS are valuable therapeutics for the treatment of bone-resorption disorders and have also demonstrated efficacy in multiple tumor types. Inhibition of human FPPS by bisphosphonates in vivo is thought to involve closing of the enzyme's C-terminal tail induced by the binding of the second substrate isopentenyl pyrophosphate (IPP). This conformational change, which occurs through a yet unclear mechanism, seals off the enzyme's active site from the solvent environment and is essential for catalysis. The crystal structure of human FPPS in complex with a novel bisphosphonate YS0470 and in the absence of a second substrate showed partial ordering of the tail in the closed conformation.
Organizational Affiliation:
Department of Biochemistry, McGill University, Montreal, Canada.