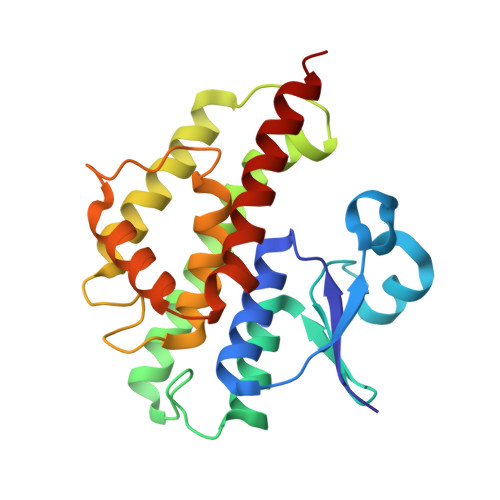
Metabolic and Target-Site Mechanisms Combine to Confer Strong DDT Resistance in Anopheles gambiae.
Mitchell, S.N., Rigden, D.J., Dowd, A.J., Lu, F., Wilding, C.S., Weetman, D., Dadzie, S., Jenkins, A.M., Regna, K., Boko, P., Djogbenou, L., Muskavitch, M.A., Ranson, H., Paine, M.J., Mayans, O., Donnelly, M.J.(2014) PLoS One 9: e92662-e92662
- PubMed: 24675797
- DOI: https://doi.org/10.1371/journal.pone.0092662
- Primary Citation of Related Structures:
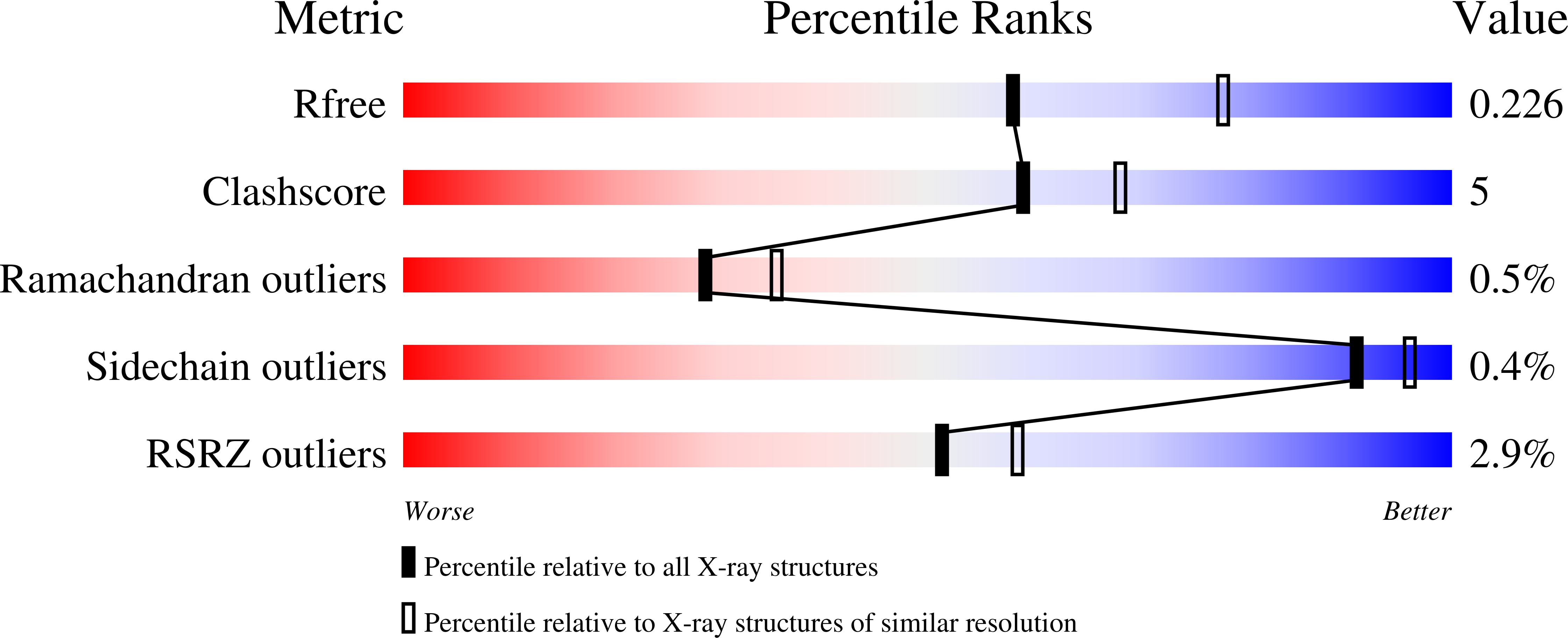
4GSN - PubMed Abstract:
The development of resistance to insecticides has become a classic exemplar of evolution occurring within human time scales. In this study we demonstrate how resistance to DDT in the major African malaria vector Anopheles gambiae is a result of both target-site resistance mechanisms that have introgressed between incipient species (the M- and S-molecular forms) and allelic variants in a DDT-detoxifying enzyme. Sequencing of the detoxification enzyme, Gste2, from DDT resistant and susceptible strains of An. gambiae, revealed a non-synonymous polymorphism (I114T), proximal to the DDT binding domain, which segregated with strain phenotype. Recombinant protein expression and DDT metabolism analysis revealed that the proteins from the susceptible strain lost activity at higher DDT concentrations, characteristic of substrate inhibition. The effect of I114T on GSTE2 protein structure was explored through X-ray crystallography. The amino acid exchange in the DDT-resistant strain introduced a hydroxyl group nearby the hydrophobic DDT-binding region. The exchange does not result in structural alterations but is predicted to facilitate local dynamics and enzyme activity. Expression of both wild-type and 114T alleles the allele in Drosophila conferred an increase in DDT tolerance. The 114T mutation was significantly associated with DDT resistance in wild caught M-form populations and acts in concert with target-site mutations in the voltage gated sodium channel (Vgsc-1575Y and Vgsc-1014F) to confer extreme levels of DDT resistance in wild caught An. gambiae.
Organizational Affiliation:
Department of Vector Biology, Liverpool School of Tropical Medicine, Liverpool, United Kingdom.