Crystal Structure of Galactose Mutarotase Galm from Bacillus Subtilis with Trehalose
Vanden Broeck, A., Sauvage, E., Herman, R., Kerff, F., Duez, C., Charlier, P.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ALDOSE 1-EPIMERASE | 324 | Bacillus subtilis subsp. subtilis str. 168 | Mutation(s): 0 Gene Names: GALM EC: 5.1.3.3 | 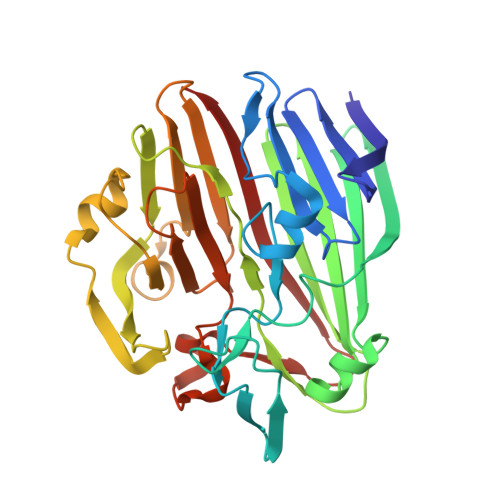 | |
UniProt | |||||
Find proteins for P39840 (Bacillus subtilis (strain 168)) Explore P39840 Go to UniProtKB: P39840 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P39840 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| CIT Query on CIT | F [auth A], G [auth B] | CITRIC ACID C6 H8 O7 KRKNYBCHXYNGOX-UHFFFAOYSA-N |  | ||
| PGE Query on PGE | E [auth A] | TRIETHYLENE GLYCOL C6 H14 O4 ZIBGPFATKBEMQZ-UHFFFAOYSA-N |  | ||
| ACT Query on ACT | H [auth B] | ACETATE ION C2 H3 O2 QTBSBXVTEAMEQO-UHFFFAOYSA-M |  | ||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| ID | Chains | Name | Type/Class | 2D Diagram | 3D Interactions |
| PRD_900006 Query on PRD_900006 | C, D | trehalose | Oligosaccharide / Nutrient |  | |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 82.19 | α = 90 |
| b = 83.322 | β = 90 |
| c = 124.446 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| MOSFLM | data reduction |
| SCALA | data scaling |
| PHASER | phasing |