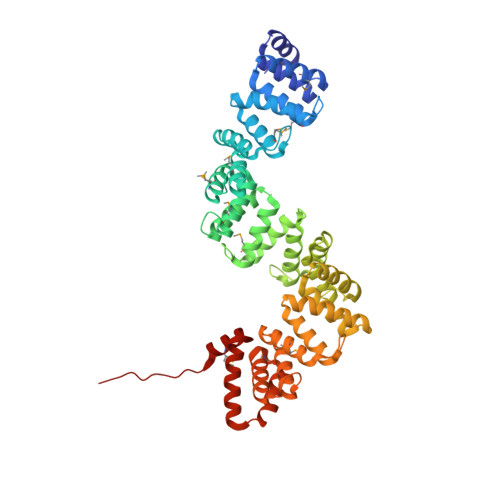
Crystal Structure of C5321: A Protective Antigen Present in Uropathogenic Escherichia Coli Strains Displaying an Slr Fold.
Urosev, D., Ferrer-Navarro, M., Pastorello, I., Cartocci, E., Costenaro, L., Zhulenkovs, D., Marechal, J., Leonchiks, A., Reverter, D., Serino, L., Soriani, M., Daura, X.(2013) BMC Struct Biol 13: 19
- PubMed: 24099525
- DOI: https://doi.org/10.1186/1472-6807-13-19
- Primary Citation of Related Structures:
4BWR - PubMed Abstract:
Increasing rates of antimicrobial resistance among uropathogens led, among other efforts, to the application of subtractive reverse vaccinology for the identification of antigens present in extraintestinal pathogenic E. coli (ExPEC) strains but absent or variable in non-pathogenic strains, in a quest for a broadly protective Escherichia coli vaccine. The protein coded by locus c5321 from CFT073 E. coli was identified as one of nine potential vaccine candidates against ExPEC and was able to confer protection with an efficacy of 33% in a mouse model of sepsis. c5321 (known also as EsiB) lacks functional annotation and structurally belongs to the Sel1-like repeat (SLR) family. Herein, as part of the general characterization of this potential antigen, we have focused on its structural properties.
Organizational Affiliation:
Institute of Biotechnology and Biomedicine, Universitat Autònoma de Barcelona, Bellaterra 08193, Spain. xavier.daura@uab.cat.