Structural basis for specific recognition of Rpt1, an ATPase subunit of the 26S proteasome, by a proteasome-dedicated chaperone Hsm3
Takagi, K., Kim, S., Yukii, H., Ueno, M., Morishita, R., Endo, Y., Kato, K., Tanaka, K., Saeki, Y., Mizushima, T.(2012) J Biol Chem 287: 12172-12182
- PubMed: 22334676
- DOI: https://doi.org/10.1074/jbc.M112.345876
- Primary Citation of Related Structures:
3VLD, 3VLE, 3VLF - PubMed Abstract:
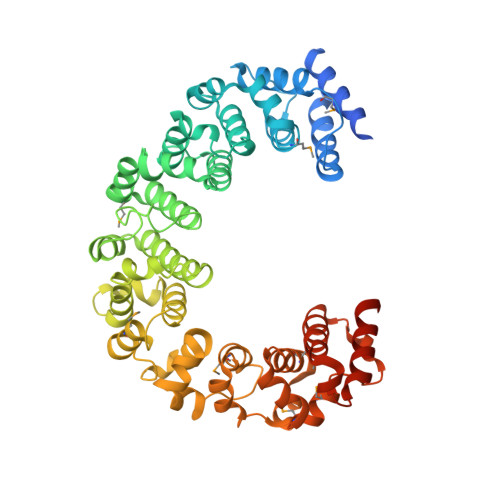
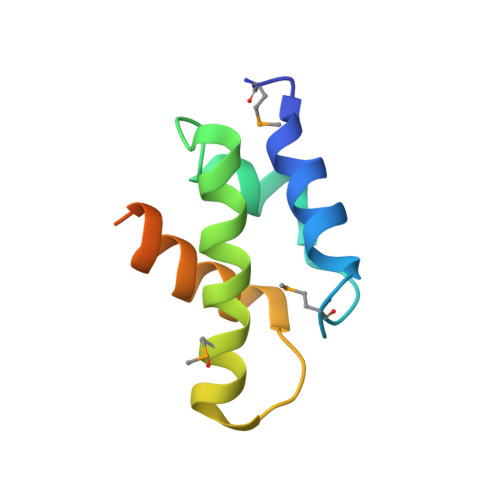
The 26 S proteasome is a 2.5-MDa molecular machine that degrades ubiquitinated proteins in eukaryotic cells. It consists of a proteolytic core particle and two 19 S regulatory particles (RPs) composed of 6 ATPase (Rpt) and 13 non-ATPase (Rpn) subunits. Multiple proteasome-dedicated chaperones facilitate the assembly of the proteasome, but little is known about the detailed mechanisms. Hsm3, a 19 S RP dedicated chaperone, transiently binds to the C-terminal domain of the Rpt1 subunit and forms a tetrameric complex, Hsm3-Rpt1-Rpt2-Rpn1, during maturation of the ATPase ring of 19 S RP. To elucidate the structural basis of Hsm3 function, we determined the crystal structures of Hsm3 and its complex with the C-terminal domain of the Rpt1 subunit (Rpt1C). Hsm3 has a C-shaped structure that consists of 11 HEAT repeats. The structure of the Hsm3-Rpt1C complex revealed that the interacting surface between Hsm3 and Rpt1 is a hydrophobic core and a complementary charged surface. Mutations in the Hsm3-Rpt1 surface resulted in the assembly defect of the 26 S proteasome. Furthermore, a structural model of the Hsm3-Rpt ring complex and an in vitro binding assay suggest that Hsm3 can bind Rpt2 in addition to Rpt1. Collectively, our results provide the structural basis of the molecular functions of Hsm3 for the RP assembly.
Organizational Affiliation:
Picobiology Institute, Department of Life Science, Graduate School of Life Science, University of Hyogo, 3-2-1, Kouto, Kamigori-cho, Ako-gun, Hyogo, 678-1297, Japan.