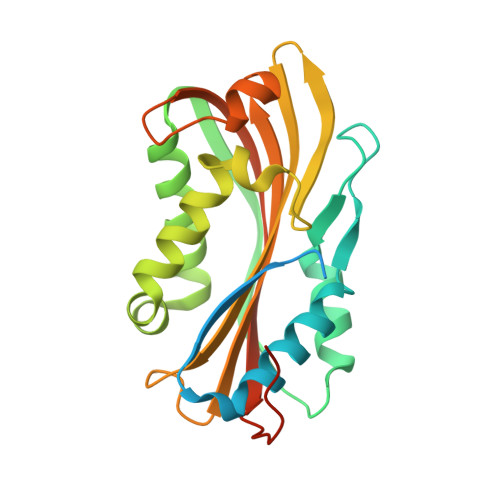
Cyt1Aa Toxin: Crystal Structure Reveals Implications for Its Membrane-Perforating Function.
Cohen, S., Albeck, S., Ben-Dov, E., Cahan, R., Firer, M., Zaritsky, A., Dym, O.(2011) J Mol Biol 413: 804-814
- PubMed: 21959261
- DOI: https://doi.org/10.1016/j.jmb.2011.09.021
- Primary Citation of Related Structures:
3RON - PubMed Abstract:
During sporulation, Bacillus thuringiensis subsp. israelensis produces a mosquito larvicidal protein complex containing several crystalline and cytolytic (Cyt) toxins. Here, the activated monomeric form of Cyt1Aa, the most toxic Cyt family member, was isolated and crystallized, and its structure was determined for the first time at 2.2 Å resolution. Cyt1Aa adopts a typical cytolysin fold containing a β-sheet held by two surrounding α-helical layers. The absence of a β-strand (between residues V26 and I37) in the dimeric structure of Cyt2Aa led us to deduce that this is the only essential segment for dimer formation and that activation of the toxin occurs by proteolytic processing of its N-terminus. Based on the Cyt1Aa structure, we suggest that the toxicity of Cyt1Aa and other nonrelated proteins, all sharing a cytolysin fold, is correlated with their ability to undergo conformational changes that are necessary prior to their membrane insertion and perforation. This fold allows the α-helical layers to swing away, exposing the β-sheet to insert into the membrane. The identification of a putative lipid binding pocket between the β-sheet and the helical layer of Cyt1Aa supports this mechanism. Sequence-based structural analysis of Cyt1Aa revealed that the lack of activity of Cyt1Ca may be related to the latter's inability to undergo this conformational change due to its lack of flexibility. The pattern of the hemolytic activity of Cyt1Aa presented here (resembling that of pore-forming agents), while differing from that imposed by ionic and nonionic detergents, further supports the pore-forming model by which conformational changes occur prior to membrane insertion and perforation.
Organizational Affiliation:
Department of Life Sciences, Ben-Gurion University of the Negev, Be'er-Sheva 84105, Israel.