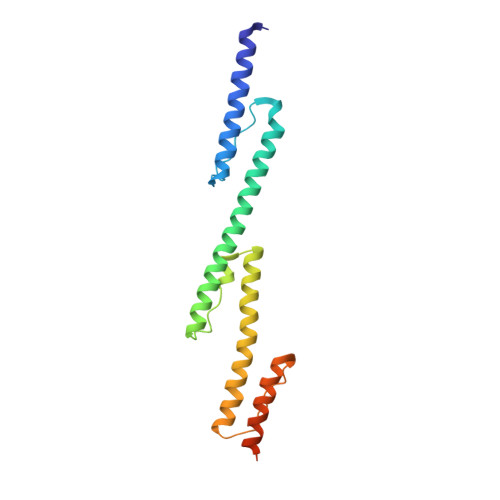
Structure of concatenated HAMP domains provides a mechanism for signal transduction.
Airola, M.V., Watts, K.J., Bilwes, A.M., Crane, B.R.(2010) Structure 18: 436-448
- PubMed: 20399181
- DOI: https://doi.org/10.1016/j.str.2010.01.013
- Primary Citation of Related Structures:
3LNR - PubMed Abstract:
HAMP domains are widespread prokaryotic signaling modules found as single domains or poly-HAMP chains in both transmembrane and soluble proteins. The crystal structure of a three-unit poly-HAMP chain from the Pseudomonas aeruginosa soluble receptor Aer2 defines a universal parallel four-helix bundle architecture for diverse HAMP domains. Two contiguous domains integrate to form a concatenated di-HAMP structure. The three HAMP domains display two distinct conformations that differ by changes in helical register, crossing angle, and rotation. These conformations are stabilized by different subsets of conserved residues. Known signals delivered to HAMP would be expected to switch the relative stability of the two conformations and the position of a coiled-coil phase stutter at the junction with downstream helices. We propose that the two conformations represent opposing HAMP signaling states and suggest a signaling mechanism whereby HAMP domains interconvert between the two states, which alternate down a poly-HAMP chain.
Organizational Affiliation:
Department of Chemistry and Chemical Biology, Cornell University, Ithaca, NY 14853, USA.