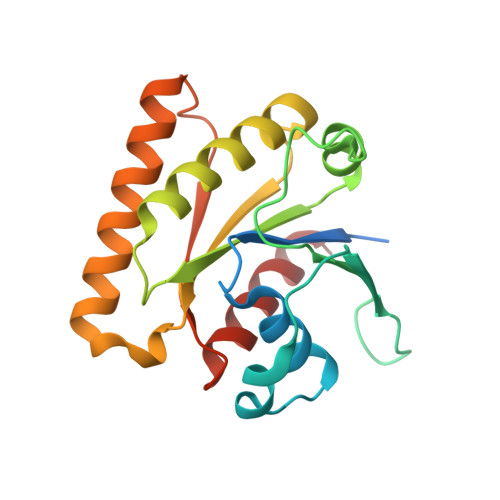
Crystal structure of the nucleotide-binding domain of Ras-related GTP-binding protein C
Nedyalkova, L., Tempel, W., Tong, Y., Crombet, L., Zhong, N., Guan, X., Arrowsmith, C.H., Edwards, A.M., Bountra, C., Weigelt, J., Bochkarev, A., Park, H.To be published.