Analysis of the HindIII-catalyzed reaction by time-resolved crystallography
Kawamura, T., Kobayashi, T., Watanabe, N.(2015) Acta Crystallogr D Biol Crystallogr 71: 256-265
- PubMed: 25664735
- DOI: https://doi.org/10.1107/S1399004714025188
- Primary Citation of Related Structures:
3WVG, 3WVH, 3WVI, 3WVK, 3WVP - PubMed Abstract:
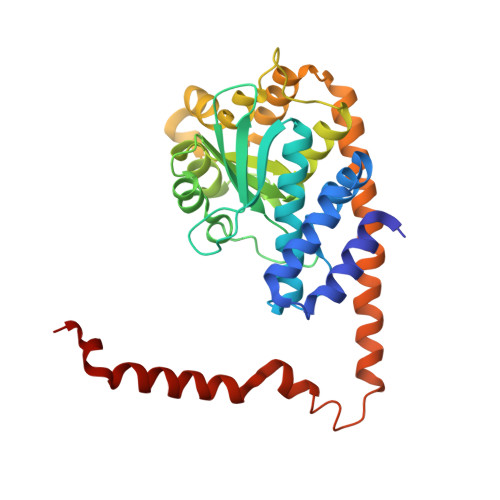
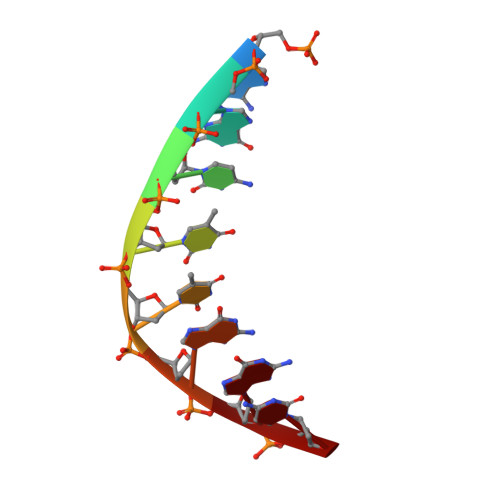
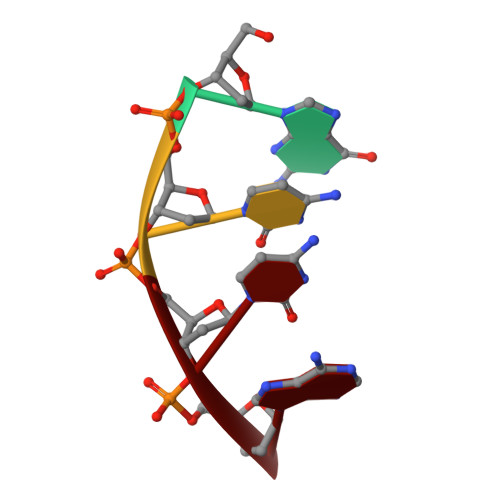
In order to investigate the mechanism of the reaction catalyzed by HindIII, structures of HindIII-DNA complexes with varying durations of soaking time in cryoprotectant buffer containing manganese ions were determined by the freeze-trap method. In the crystal structures of the complexes obtained after soaking for a longer duration, two manganese ions, indicated by relatively higher electron density, are clearly observed at the two metal ion-binding sites in the active site of HindIII. The increase in the electron density of the two metal-ion peaks followed distinct pathways with increasing soaking times, suggesting variation in the binding rate constant for the two metal sites. DNA cleavage is observed when the second manganese ion appears, suggesting that HindIII uses the two-metal-ion mechanism, or alternatively that its reactivity is enhanced by the binding of the second metal ion. In addition, conformational change in a loop near the active site accompanies the catalytic reaction.
Organizational Affiliation:
Synchrotron Radiation Research Center, Nagoya University, Chikusa-ku, Nagoya 464-8603, Japan.