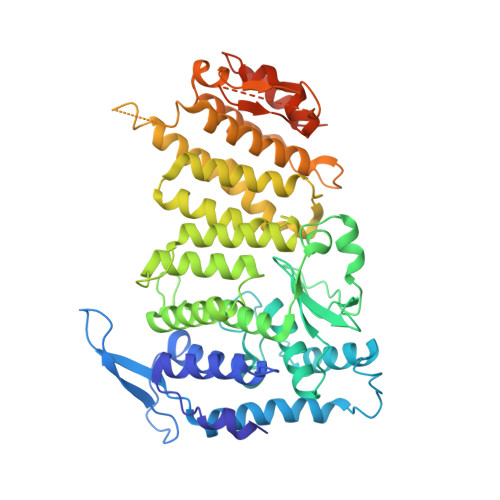
The same pocket in menin binds both MLL and JUND but has opposite effects on transcription.
Huang, J., Gurung, B., Wan, B., Matkar, S., Veniaminova, N.A., Wan, K., Merchant, J.L., Hua, X., Lei, M.(2012) Nature 482: 542-546
- PubMed: 22327296
- DOI: https://doi.org/10.1038/nature10806
- Primary Citation of Related Structures:
3U84, 3U85, 3U86, 3U88 - PubMed Abstract:
Menin is a tumour suppressor protein whose loss or inactivation causes multiple endocrine neoplasia 1 (MEN1), a hereditary autosomal dominant tumour syndrome that is characterized by tumorigenesis in multiple endocrine organs. Menin interacts with many proteins and is involved in a variety of cellular processes. Menin binds the JUN family transcription factor JUND and inhibits its transcriptional activity. Several MEN1 missense mutations disrupt the menin-JUND interaction, suggesting a correlation between the tumour-suppressor function of menin and its suppression of JUND-activated transcription. Menin also interacts with mixed lineage leukaemia protein 1 (MLL1), a histone H3 lysine 4 methyltransferase, and functions as an oncogenic cofactor to upregulate gene transcription and promote MLL1-fusion-protein-induced leukaemogenesis. A recent report on the tethering of MLL1 to chromatin binding factor lens epithelium-derived growth factor (LEDGF) by menin indicates that menin is a molecular adaptor coordinating the functions of multiple proteins. Despite its importance, how menin interacts with many distinct partners and regulates their functions remains poorly understood. Here we present the crystal structures of human menin in its free form and in complexes with MLL1 or with JUND, or with an MLL1-LEDGF heterodimer. These structures show that menin contains a deep pocket that binds short peptides of MLL1 or JUND in the same manner, but that it can have opposite effects on transcription. The menin-JUND interaction blocks JUN N-terminal kinase (JNK)-mediated JUND phosphorylation and suppresses JUND-induced transcription. In contrast, menin promotes gene transcription by binding the transcription activator MLL1 through the peptide pocket while still interacting with the chromatin-anchoring protein LEDGF at a distinct surface formed by both menin and MLL1.
Organizational Affiliation:
Howard Hughes Medical Institute, University of Michigan Medical School, Ann Arbor, Michigan 48109, USA.