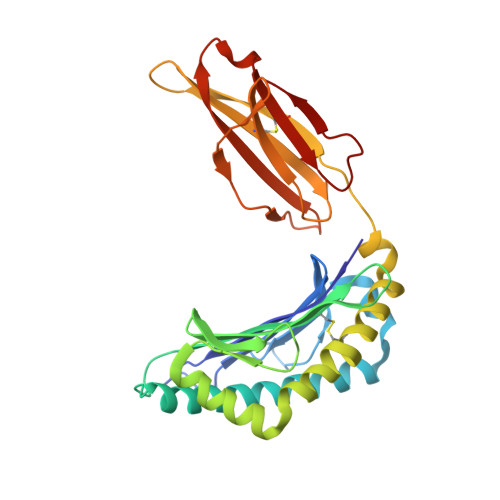
Nitro-tyrosination of the immunodominant LCMV epitope gp34-41 alters both its capacity to stabilize H-2Kb and the molecular surface of the MHC complex, affecting TCR recognition
Madhurantakam, C., Duru, A.D., Leong, C., Sandalova, T., Webb, J.R., Achour, A.(2012) PLoS One