Of bits and bugs--on the use of bioinformatics and a bacterial crystal structure to solve a eukaryotic repeat-protein structure.
Graebsch, A., Roche, S., Kostrewa, D., Soding, J., Niessing, D.(2010) PLoS One 5: e13402-e13402
- PubMed: 20976240
- DOI: https://doi.org/10.1371/journal.pone.0013402
- Primary Citation of Related Structures:
3N8B - PubMed Abstract:
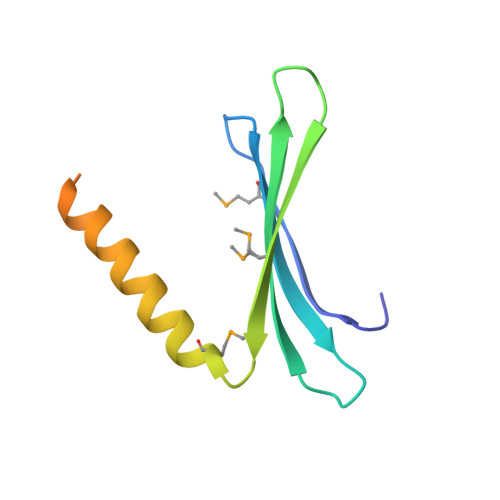
Pur-α is a nucleic acid-binding protein involved in cell cycle control, transcription, and neuronal function. Initially no prediction of the three-dimensional structure of Pur-α was possible. However, recently we solved the X-ray structure of Pur-α from the fruitfly Drosophila melanogaster and showed that it contains a so-called PUR domain. Here we explain how we exploited bioinformatics tools in combination with X-ray structure determination of a bacterial homolog to obtain diffracting crystals and the high-resolution structure of Drosophila Pur-α. First, we used sensitive methods for remote-homology detection to find three repetitive regions in Pur-α. We realized that our lack of understanding how these repeats interact to form a globular domain was a major problem for crystallization and structure determination. With our information on the repeat motifs we then identified a distant bacterial homolog that contains only one repeat. We determined the bacterial crystal structure and found that two of the repeats interact to form a globular domain. Based on this bacterial structure, we calculated a computational model of the eukaryotic protein. The model allowed us to design a crystallizable fragment and to determine the structure of Drosophila Pur-α. Key for success was the fact that single repeats of the bacterial protein self-assembled into a globular domain, instructing us on the number and boundaries of repeats to be included for crystallization trials with the eukaryotic protein. This study demonstrates that the simpler structural domain arrangement of a distant prokaryotic protein can guide the design of eukaryotic crystallization constructs. Since many eukaryotic proteins contain multiple repeats or repeating domains, this approach might be instructive for structural studies of a range of proteins.
Organizational Affiliation:
Institute of Structural Biology, Helmholtz Zentrum München, Munich, Germany.