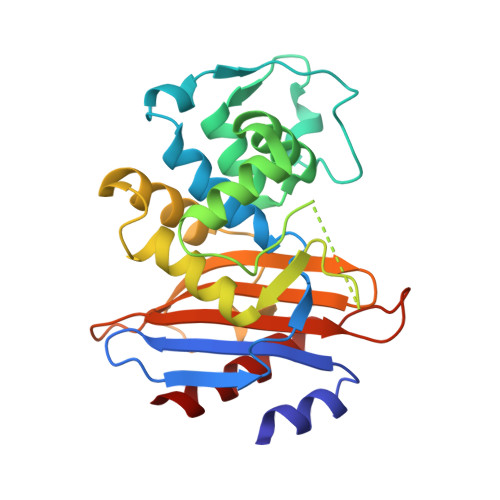
Structural studies of the mechanism for biosensing antibiotics in a fluorescein-labeled beta-lactamase.
Wong, W.T., Au, H.W., Yap, H.K., Leung, Y.C., Wong, K.Y., Zhao, Y.(2011) BMC Struct Biol 11: 15-15
- PubMed: 21443768
- DOI: https://doi.org/10.1186/1472-6807-11-15
- Primary Citation of Related Structures:
3M2K - PubMed Abstract:
β-lactamase conjugated with environment-sensitive fluorescein molecule to residue 166 on the Ω-loop near its catalytic site is a highly effective biosensor for β-lactam antibiotics. Yet the molecular mechanism of such fluorescence-based biosensing is not well understood.
Organizational Affiliation:
Department of Applied Biology and Chemical Technology, Central Laboratory of Institute of Molecular Technology for Drug Discovery and Synthesis, The Hong Kong Polytechnic University, Hung Hom, Hong Hong, China.