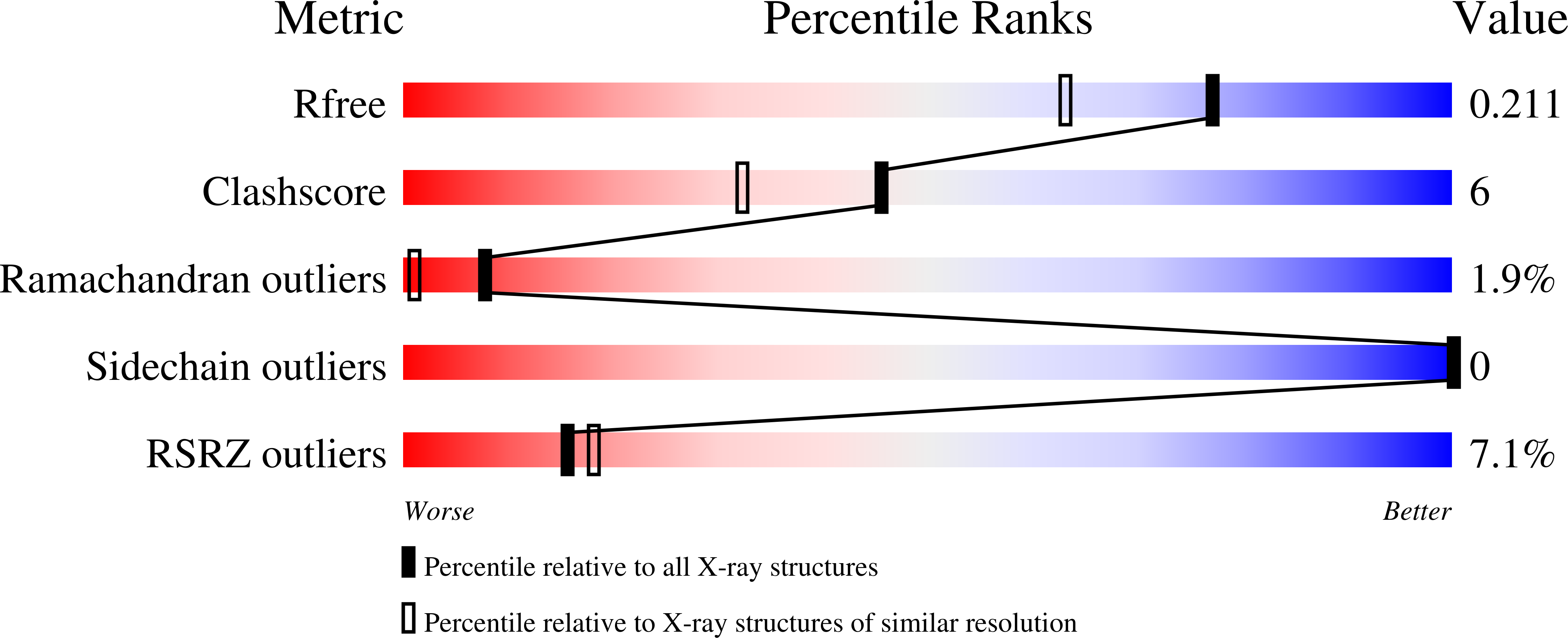
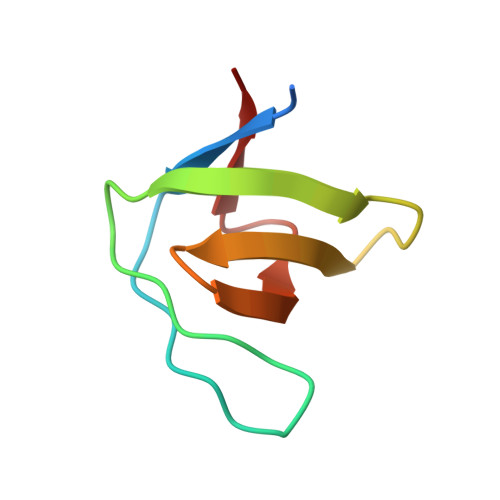
Understanding the polymorphic behaviour of a mutant of the alpha-spectrin SH3 domain by means of two 1.1 A structures
Camara-Artigas, A., Gavira, J.A., Casares, S., Garcia-Ruiz, J.M., Conejero-Lara, F., Allen, J.P., Martinez, J.(2011) Acta Crystallogr D Biol Crystallogr