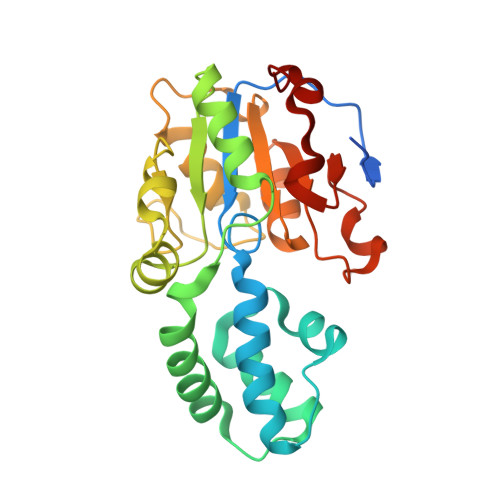
The crystal structure of human Haloacid Dehalogenase-like Hydrolase Domain containing 1A (HDHD1A)
Ugochukwu, E., Guo, K., Picaud, S., Muniz, J., Allerston, C., von Delft, F., Bountra, C., Arrowsmith, C.H., Weigelt, J., Edwards, A., Yue, W.W., Kavanagh, K.L., Oppermann, U., Structural Genomics Consortium (SGC)To be published.