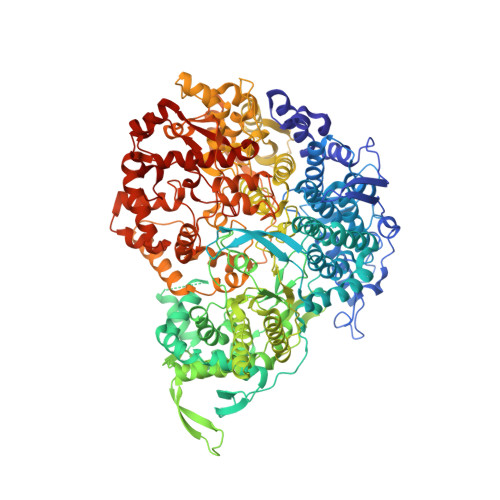
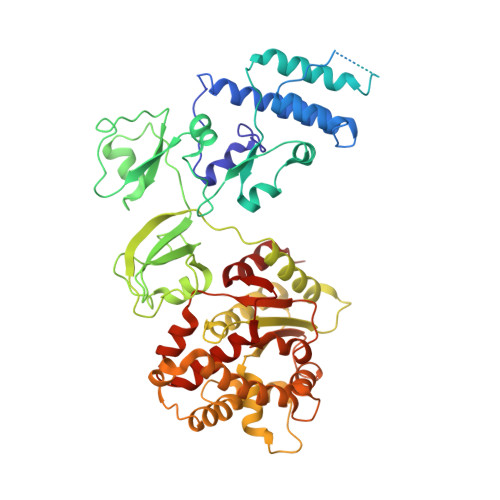
In situ structures of the segmented genome and RNA polymerase complex inside a dsRNA virus.
Zhang, X., Ding, K., Yu, X., Chang, W., Sun, J., Hong Zhou, Z.(2015) Nature 527: 531-534
- PubMed: 26503045
- DOI: https://doi.org/10.1038/nature15767
- Primary Citation of Related Structures:
3JB6, 3JB7 - PubMed Abstract:
Viruses in the Reoviridae, like the triple-shelled human rotavirus and the single-shelled insect cytoplasmic polyhedrosis virus (CPV), all package a genome of segmented double-stranded RNAs (dsRNAs) inside the viral capsid and carry out endogenous messenger RNA synthesis through a transcriptional enzyme complex (TEC). By direct electron-counting cryoelectron microscopy and asymmetric reconstruction, we have determined the organization of the dsRNA genome inside quiescent CPV (q-CPV) and the in situ atomic structures of TEC within CPV in both quiescent and transcribing (t-CPV) states. We show that the ten segmented dsRNAs in CPV are organized with ten TECs in a specific, non-symmetric manner, with each dsRNA segment attached directly to a TEC. The TEC consists of two extensively interacting subunits: an RNA-dependent RNA polymerase (RdRP) and an NTPase VP4. We find that the bracelet domain of RdRP undergoes marked conformational change when q-CPV is converted to t-CPV, leading to formation of the RNA template entry channel and access to the polymerase active site. An amino-terminal helix from each of two subunits of the capsid shell protein (CSP) interacts with VP4 and RdRP. These findings establish the link between sensing of environmental cues by the external proteins and activation of endogenous RNA transcription by the TEC inside the virus.
Organizational Affiliation:
California Nanosystems Institute, Los Angeles, CA 90095, USA.