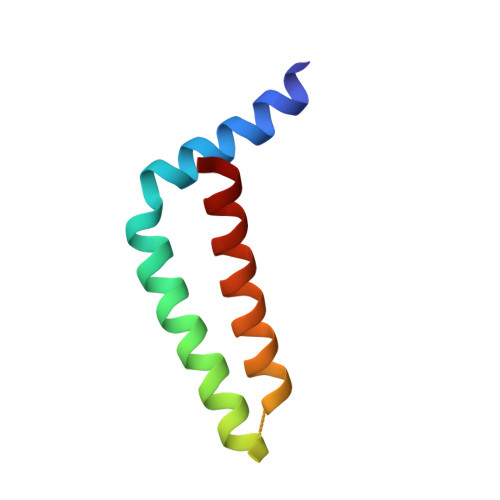
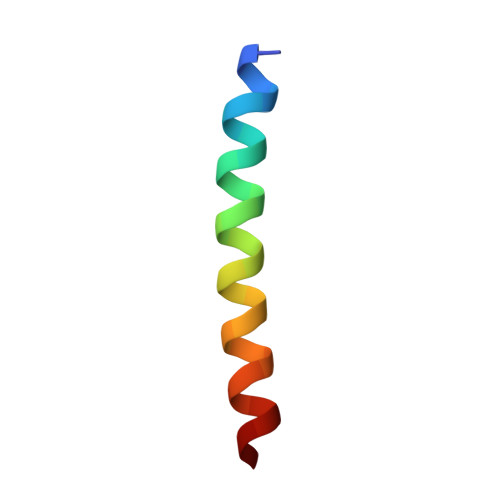
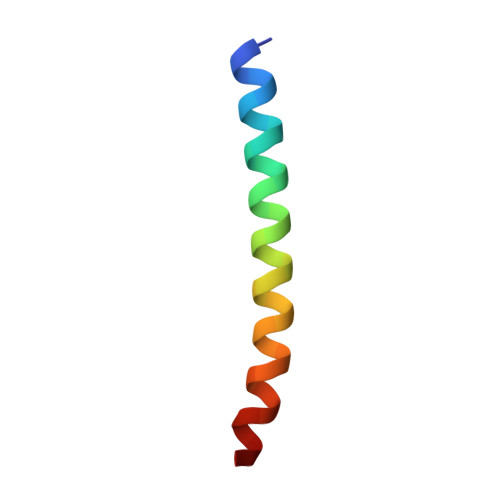
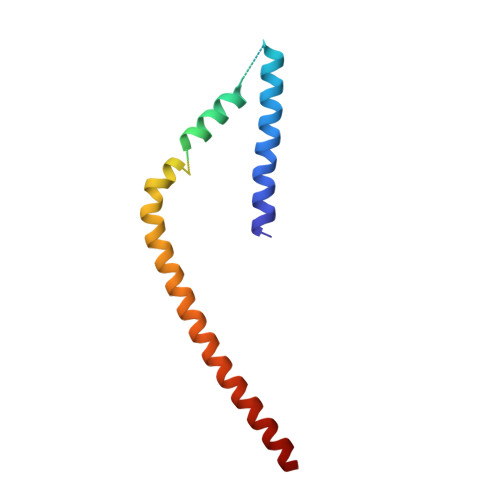Formation of an Intricate Helical Bundle Dictates the Assembly of the 26S Proteasome Lid.
Estrin, E., Lopez-Blanco, J.R., Chacon, P., Martin, A.(2013) Structure 21: 1624-1635
- PubMed: 23911091
- DOI: https://doi.org/10.1016/j.str.2013.06.023
- Primary Citation of Related Structures:
3J47 - PubMed Abstract:
The 26S proteasome is the major ATP-dependent protease in eukaryotes and thus involved in regulating a diverse array of vital cellular processes. Three subcomplexes form this massive degradation machine: the lid, the base, and the core. While assembly of base and core has been well-studied, the detailed molecular mechanisms involved in formation of the nine-subunit lid remain largely unknown. Here, we reveal that helices found at the C terminus of each lid subunit form a helical bundle that directs the ordered self-assembly of the lid subcomplex. Furthermore, we use an integrative modeling approach to gain critical insights into the bundle topology and provide an important structural framework for our biochemical data. We show that the helical bundle serves as a hub through which the last-added subunit Rpn12 monitors proper lid assembly before incorporation into the proteasome. Finally, we predict that the assembly of the COP9 signalosome depends on a similar helical bundle.
Organizational Affiliation:
Department of Molecular and Cell Biology, University of California, Berkeley, Berkeley, CA 94720, USA.