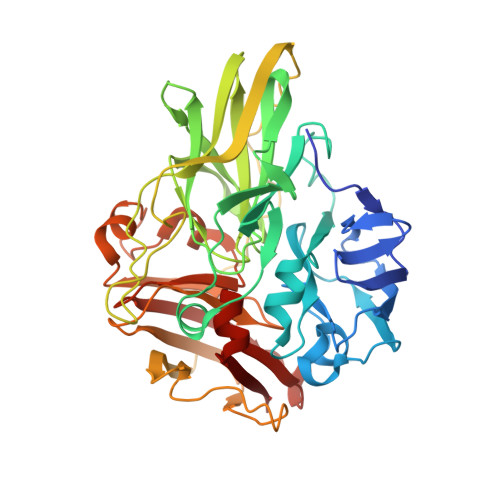
Structure of a multicopper oxidase from the hyperthermophilic archaeon Pyrobaculum aerophilum
Sakuraba, H., Koga, K., Yoneda, K., Kashima, Y., Ohshima, T.(2011) Acta Crystallogr Sect F Struct Biol Cryst Commun 67: 753-757
- PubMed: 21795787
- DOI: https://doi.org/10.1107/S1744309111018173
- Primary Citation of Related Structures:
3AW5 - PubMed Abstract:
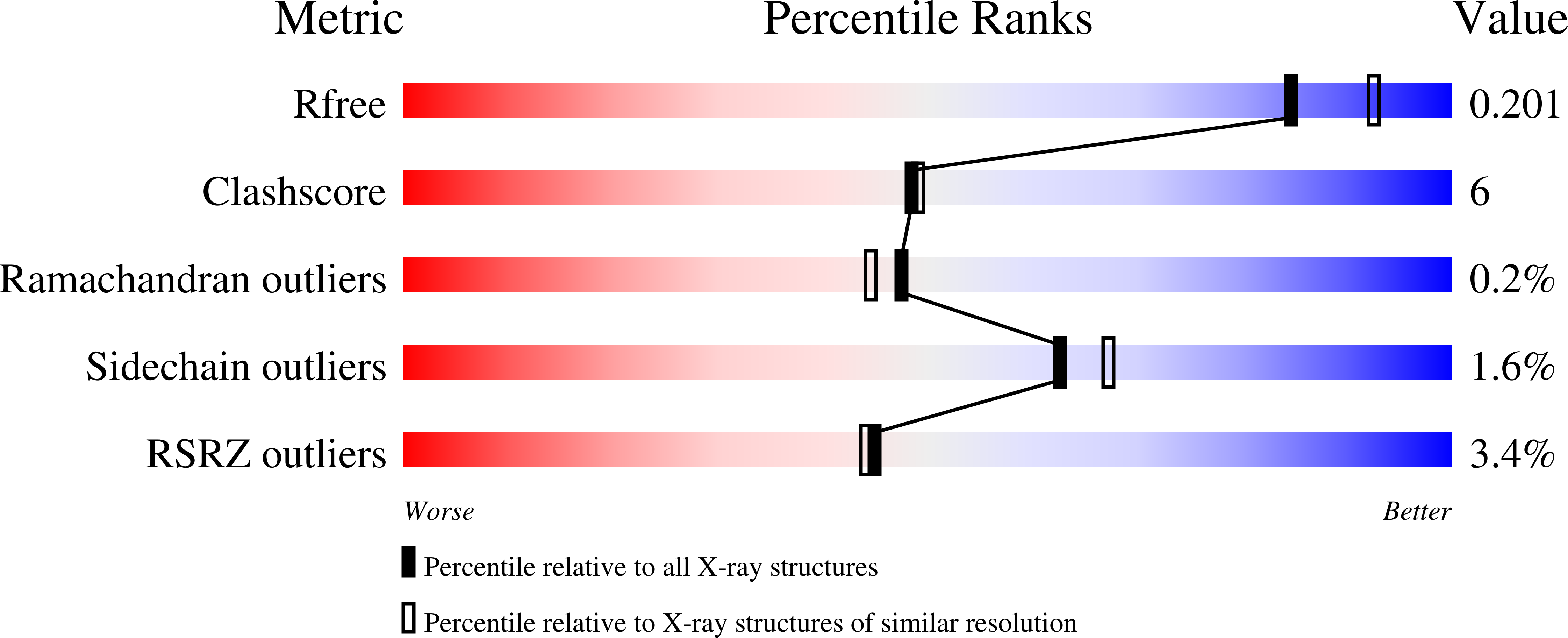
The crystal structure of an extremely thermostable multicopper oxidase (McoP) from the hyperthermophilic archaeon Pyrobaculum aerophilum was determined at a resolution of 2.0 Å. The overall fold was comprised of three cupredoxin-like domains and the main-chain coordinates of the enzyme were similar to those of multicopper oxidases from Escherichia coli (CueO) and Bacillus subtilis (CotA). However, there were clear topological differences around domain 3 between McoP and the other two enzymes: a methionine-rich helix in CueO and a protruding helix in CotA were not present in McoP. Instead, a large loop (PL-1) covered the T1 copper centre of McoP and a short α-helix in domain 3 extended near the N-terminal end of PL-1. In addition, the sizes of several surface loops in McoP were markedly smaller than the corresponding loops in CueO and CotA. Structural comparison revealed that the presence of extensive hydrophobic interactions and a smaller cavity volume are likely to be the main factors contributing to the hyperthermostability of McoP.
Organizational Affiliation:
Department of Applied Biological Science, Faculty of Agriculture, Kagawa University, 2393 Ikenobe, Miki-cho, Kita-gun, Kagawa 761-0795, Japan. sakuraba@ag.kagawa-u.ac.jp