Novel Fragments of Clavulanate Observed in the Structure of the Class a Beta-Lactamase from Bacillus Licheniformis Bs3.
Power, P., Mercuri, P., Herman, R., Kerff, F., Gutkind, G., Dive, G., Galleni, M., Charlier, P., Sauvage, E.(2012) J Antimicrob Chemother 67: 2379
- PubMed: 22773738
- DOI: https://doi.org/10.1093/jac/dks231
- Primary Citation of Related Structures:
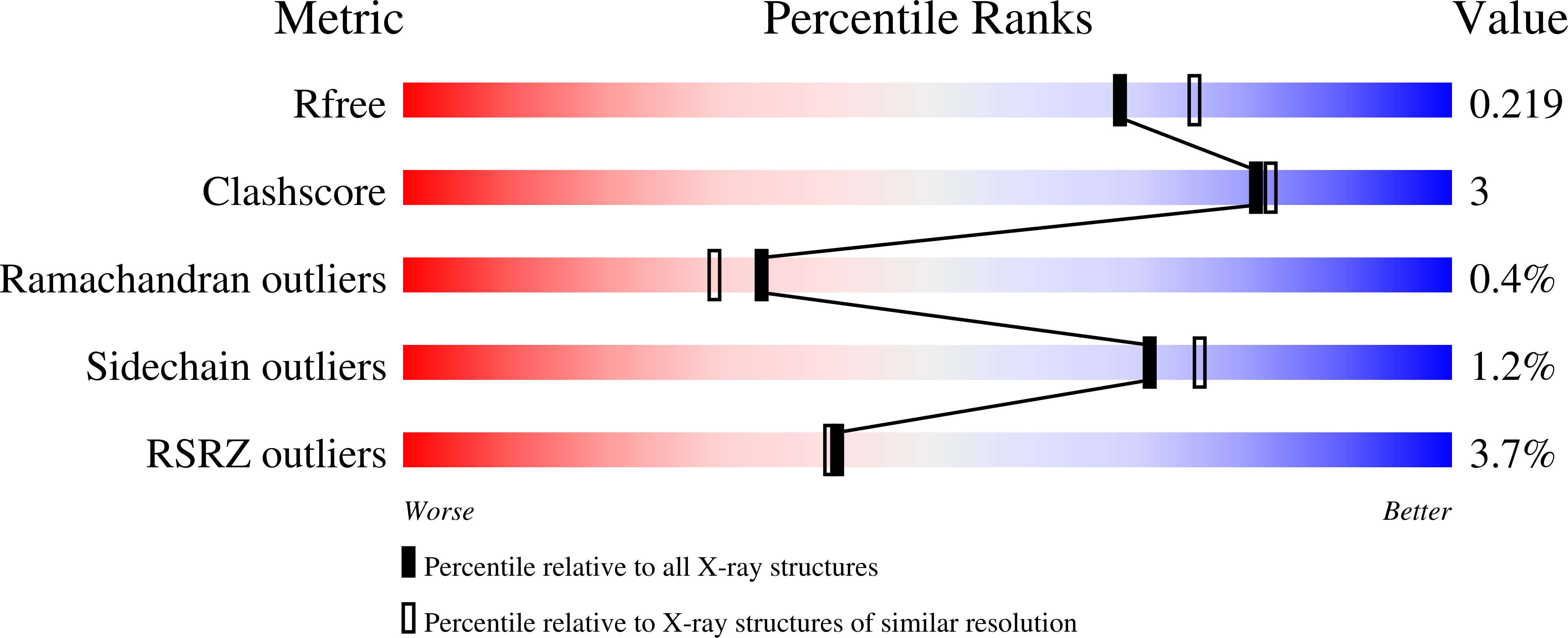
2Y91 - PubMed Abstract:
Our aim was to unravel the inactivation pathway of the class A β-lactamase produced by Bacillus licheniformis BS3 (BS3) by clavulanate.
Organizational Affiliation:
Laboratorio de Resistencia Bacteriana, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Junin 956, (1113) Buenos Aires, Argentina.