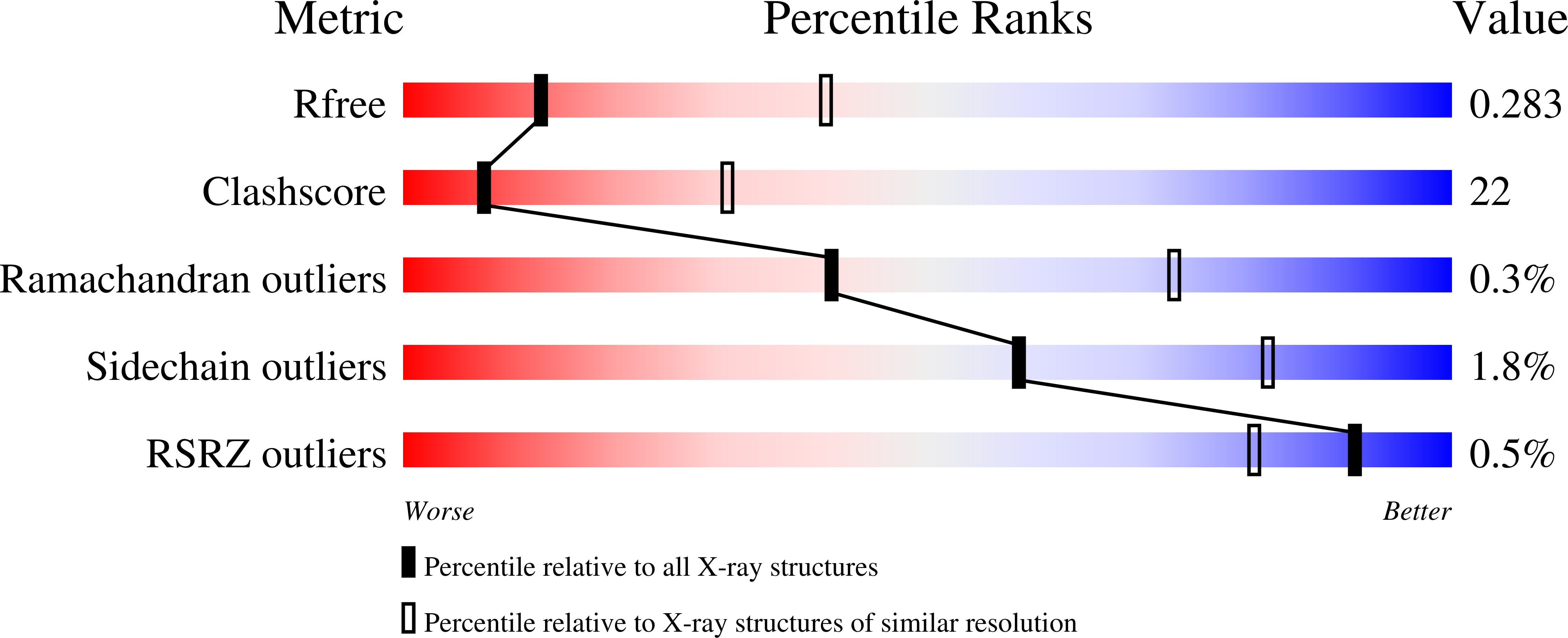
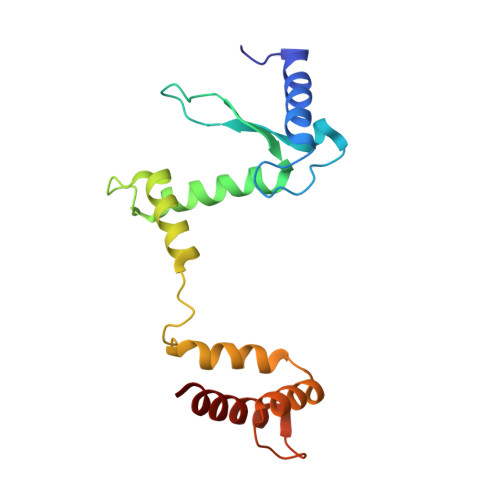
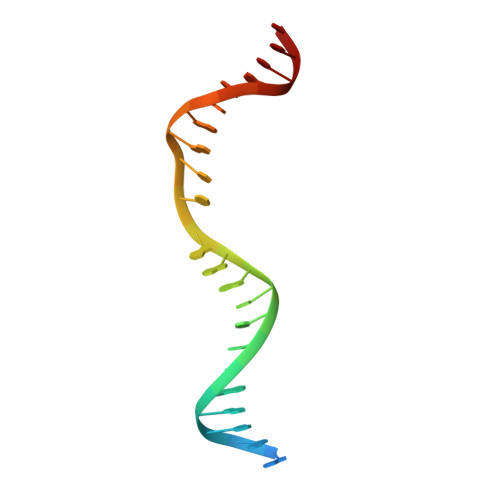
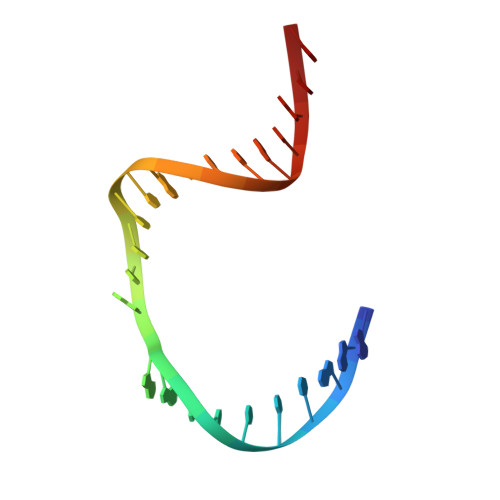Crystal structure of T4 endonuclease VII resolving a Holliday junction.
Biertumpfel, C., Yang, W., Suck, D.(2007) Nature 449: 616-620
- PubMed: 17873859
- DOI: https://doi.org/10.1038/nature06152
- Primary Citation of Related Structures:
2QNC, 2QNF - PubMed Abstract:
Holliday proposed a four-way DNA junction as an intermediate in homologous recombination, and such Holliday junctions have since been identified as a central component in DNA recombination and repair. Phage T4 endonuclease VII (endo VII) was the first enzyme shown to resolve Holliday junctions into duplex DNAs by introducing symmetrical nicks in equivalent strands. Several Holliday junction resolvases have since been characterized, but an atomic structure of a resolvase complex with a Holliday junction remained elusive. Here we report the crystal structure of an inactive T4 endo VII(N62D) complexed with an immobile four-way junction with alternating arm lengths of 10 and 14 base pairs. The junction is a hybrid of the conventional square-planar and stacked-X conformation. Endo VII protrudes into the junction point from the minor groove side, opening it to a 14 A x 32 A parallelogram. This interaction interrupts the coaxial stacking, yet every base pair surrounding the junction remains intact. Additional interactions involve the positively charged protein and DNA phosphate backbones. Each scissile phosphate that is two base pairs from the crossover interacts with a Mg2+ ion in the active site. The similar overall shape and surface charge potential of the Holliday junction resolvases endo VII, RuvC, Ydc2, Hjc and RecU, despite having different folds, active site composition and DNA sequence preference, suggest a conserved binding mode for Holliday junctions.
Organizational Affiliation:
National Institute of Diabetes and Digestive and Kidney Diseases, Laboratory of Molecular Biology, 9000 Rockville Pike, Bethesda, Maryland 20892, USA.