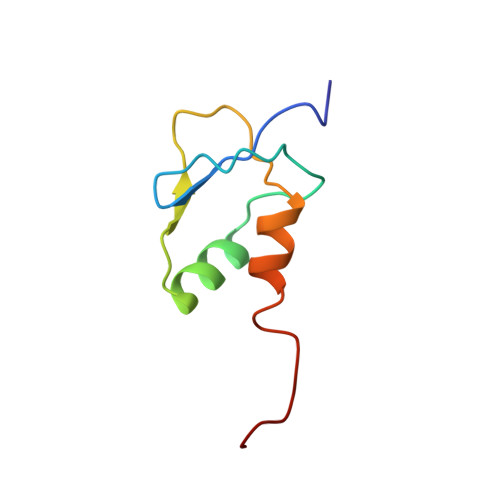
Solution structure of a novel zinc finger motif in the SAP30 polypeptide of the Sin3 corepressor complex and its potential role in nucleic acid recognition
He, Y., Imhoff, R., Sahu, A., Radhakrishnan, I.(2009) Nucleic Acids Res 37: 2142-2152
- PubMed: 19223330
- DOI: https://doi.org/10.1093/nar/gkp051
- Primary Citation of Related Structures:
2KDP - PubMed Abstract:
Giant chromatin-modifying complexes regulate gene transcription in eukaryotes by acting on chromatin substrates and 'setting' the histone code. The histone deacetylase (HDAC)-associated mammalian Sin3 corepressor complex regulates a wide variety of genes involved in all aspects of cellular physiology. The recruitment of the corepressor complex by transcription factors to specific regions of the genome is mediated by Sin3 as well as 10 distinct polypeptides that comprise the corepressor complex. Here we report the solution structure of a novel CCCH zinc finger (ZnF) motif in the SAP30 polypeptide, a key component of the corepressor complex. The structure represents a novel fold comprising two beta-strands and two alpha-helices with the zinc organizing center showing remote resemblance to the treble clef motif. In silico analysis of the structure revealed a highly conserved surface that is dominated by basic residues. NMR-based analysis of potential ligands for the SAP30 ZnF motif indicated a strong preference for nucleic acid substrates. We propose that the SAP30 ZnF functions as a double-stranded DNA-binding motif, thereby expanding the known functions of both SAP30 and the mammalian Sin3 corepressor complex. Our results also call into question the common assumption about the exclusion of DNA-binding core subunits within chromatin-modifying/remodeling complexes.
Organizational Affiliation:
Department of Biochemistry, Molecular Biology, and Cell Biology, Northwestern University, Evanston, IL 60208-3500, USA.