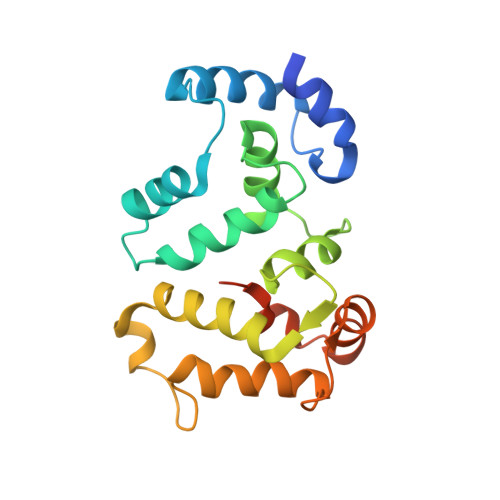
Structural Basis for Calcium-induced Inhibition of Rhodopsin Kinase by Recoverin.
Ames, J.B., Levay, K., Wingard, J.N., Lusin, J.D., Slepak, V.Z.(2006) J Biol Chem 281: 37237-37245
- PubMed: 17020884
- DOI: https://doi.org/10.1074/jbc.M606913200
- Primary Citation of Related Structures:
2I94 - PubMed Abstract:
Recoverin, a member of the neuronal calcium sensor branch of the EF-hand superfamily, serves as a calcium sensor that regulates rhodopsin kinase (RK) activity in retinal rod cells. We report here the NMR structure of Ca(2+)-bound recoverin bound to a functional N-terminal fragment of rhodopsin kinase (residues 1-25, called RK25). The overall main-chain structure of recoverin in the complex is similar to structures of Ca(2+)-bound recoverin in the absence of target (<1.8A root-mean-square deviation). The first eight residues of recoverin at the N terminus are solvent-exposed, enabling the N-terminal myristoyl group to interact with target membranes, and Ca(2+) is bound at the second and third EF-hands of the protein. RK25 in the complex forms an amphipathic helix (residues 4-16). The hydrophobic face of the RK25 helix (Val-9, Val-10, Ala-11, Ala-14, and Phe-15) interacts with an exposed hydrophobic groove on the surface of recoverin lined by side-chain atoms of Trp-31, Phe-35, Phe-49, Ile-52, Tyr-53, Phe-56, Phe-57, Tyr-86, and Leu-90. Residues of recoverin that contact RK25 are highly conserved, suggesting a similar target binding site structure in all neuronal calcium sensor proteins. Site-specific mutagenesis and deletion analysis confirm that the hydrophobic residues at the interface are necessary and sufficient for binding. The recoverin-RK25 complex exhibits Ca(2+)-induced binding to rhodopsin immobilized on concanavalin-A resin. We propose that Ca(2+)-bound recoverin is bound between rhodopsin and RK in a ternary complex on rod outer segment disk membranes, thereby blocking RK interaction with rhodopsin at high Ca(2+).
Organizational Affiliation:
Center for Advanced Research in Biotechnology, University of Maryland Biotechnology Institute, Rockville, Maryland 20850, USA. ames@chem.ucdavis.edu