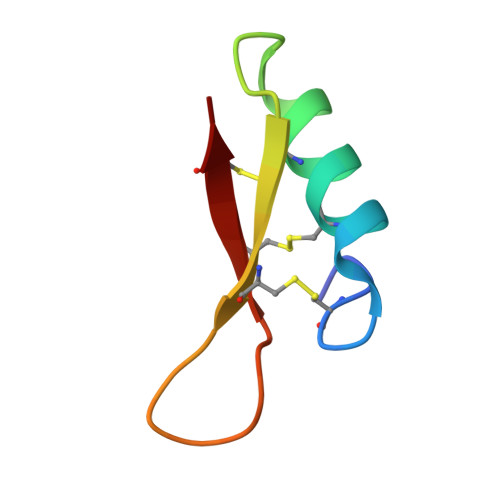
Eurocin, a New Fungal Defensin: STRUCTURE, LIPID BINDING, AND ITS MODE OF ACTION.
Oeemig, J.S., Lynggaard, C., Knudsen, D.H., Hansen, F.T., Norgaard, K.D., Schneider, T., Vad, B.S., Sandvang, D.H., Nielsen, L.A., Neve, S., Kristensen, H.H., Sahl, H.G., Otzen, D.E., Wimmer, R.(2012) J Biol Chem 287: 42361-42372
- PubMed: 23093408
- DOI: https://doi.org/10.1074/jbc.M112.382028
- Primary Citation of Related Structures:
2LT8 - PubMed Abstract:
Antimicrobial peptides are a new class of antibiotics that are promising for pharmaceutical applications because they have retained efficacy throughout evolution. One class of antimicrobial peptides are the defensins, which have been found in different species. Here we describe a new fungal defensin, eurocin. Eurocin acts against a range of Gram-positive human pathogens but not against Gram-negative bacteria. Eurocin consists of 42 amino acids, forming a cysteine-stabilized α/β-fold. The thermal denaturation data point shows the disulfide bridges being responsible for the stability of the fold. Eurocin does not form pores in cell membranes at physiologically relevant concentrations; it does, however, lead to limited leakage of a fluorophore from small unilamellar vesicles. Eurocin interacts with detergent micelles, and it inhibits the synthesis of cell walls by binding equimolarly to the cell wall precursor lipid II.
Organizational Affiliation:
Department of Biotechnology, Chemistry, and Environmental Engineering, Aalborg University, Sohngaardsholmsvej 49, DK-9000 Aalborg, Denmark.