Probing the conformation and dynamics of allatostatin neuropeptides: a structural model for functional differences.
Banerjee, M., Meyerowitz, E., Huang, C., Mohanty, S.(2008) Peptides 29: 375-385
- PubMed: 18191874
- DOI: https://doi.org/10.1016/j.peptides.2007.11.016
- Primary Citation of Related Structures:
2JQS, 2JQU - PubMed Abstract:
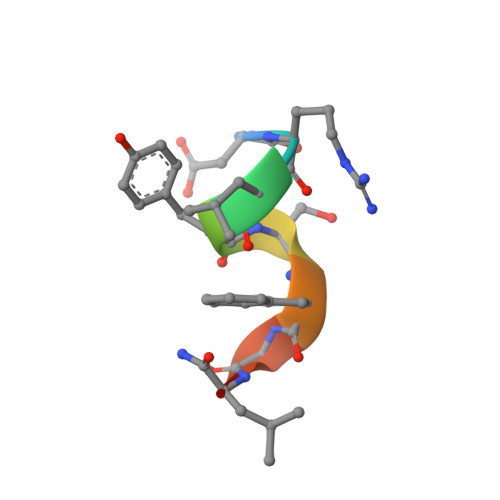
Allatostatins are a family of related neuropeptides that play an important role in development, reproduction, and digestion in insects. The cockroach Diploptera punctata has 13 allatostatin neuropeptides, with pleiotropic functions, two of which are: inhibition of juvenile hormone (JH) production and inhibition of gut muscle contraction. In this study, the conformation and dynamics of D. punctata allatostatin 5 (Dippu-AST 5) and allatostatin 8 (Dippu-AST 8) are investigated by CD, NMR, and molecular dynamics simulations. These peptides contain eight and nine residues, respectively, and the identical six-residue C-terminal motif. Yet Dippu-AST 5 and Dippu-AST 8 affect juvenile hormone production and hindgut contraction with different potencies. Dippu-AST 5 is one of the most potent inhibitors of juvenile hormone production and one of the least potent inhibitors of gut contraction, whereas Dippu-AST 8 has the opposite potencies with respect to these tissues. From the NMR structure, it is clear that Dippu-AST 5 has a 3(10) helix involving three of its residues and a "gamma" turn at the end of its C-terminal motif. In contrast Dippu-AST 8 has an open "pi" turn among five of its central residues. In addition, the orientation preferences within the membrane of the two peptides were simulated. Our simulation results show that the C-terminal segment of Dippu-AST 5 orients in the membrane surface with an average angle of 17.5 degrees, whereas Dippu-AST 8 orients with an average angle of 5.1 degrees. Taken together, from the structures and orientation preferences of these peptides within the membrane, it appears that these peptides may interact with the receptor very differently.
Organizational Affiliation:
Department of Chemistry and Biochemistry, Auburn University, Auburn, AL 36849-5312, USA.