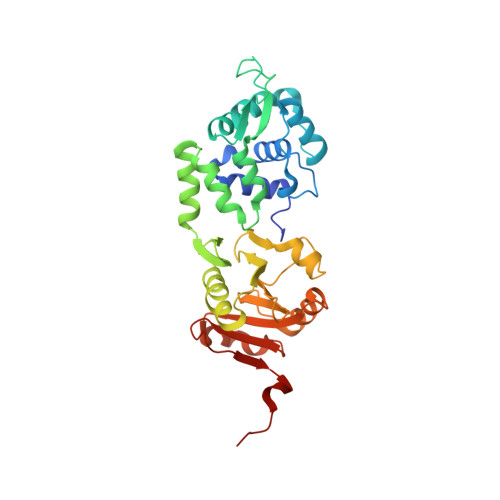
The Crystal Structure of the Rare-Cutting Restriction Enzyme Sdai Reveals Unexpected Domain Architecture
Tamulaitiene, G., Jakubauskas, A., Urbanke, C., Huber, R., Grazulis, S., Siksnys, V.(2006) Structure 14: 1389
- PubMed: 16962970
- DOI: https://doi.org/10.1016/j.str.2006.07.002
- Primary Citation of Related Structures:
2IXS - PubMed Abstract:
Rare-cutting restriction enzymes are important tools in genome analysis. We report here the crystal structure of SdaI restriction endonuclease, which is specific for the 8 bp sequence CCTGCA/GG ("/" designates the cleavage site). Unlike orthodox Type IIP enzymes, which are single domain proteins, the SdaI monomer is composed of two structural domains. The N domain contains a classical winged helix-turn-helix (wHTH) DNA binding motif, while the C domain shows a typical restriction endonuclease fold. The active site of SdaI is located within the C domain and represents a variant of the canonical PD-(D/E)XK motif. SdaI determinants of sequence specificity are clustered on the recognition helix of the wHTH motif at the N domain. The modular architecture of SdaI, wherein one domain mediates DNA binding while the other domain is predicted to catalyze hydrolysis, distinguishes SdaI from previously characterized restriction enzymes interacting with symmetric recognition sequences.
Organizational Affiliation:
Institute of Biotechnology, Graiciuno 8, LT-02241 Vilnius, Lithuania.