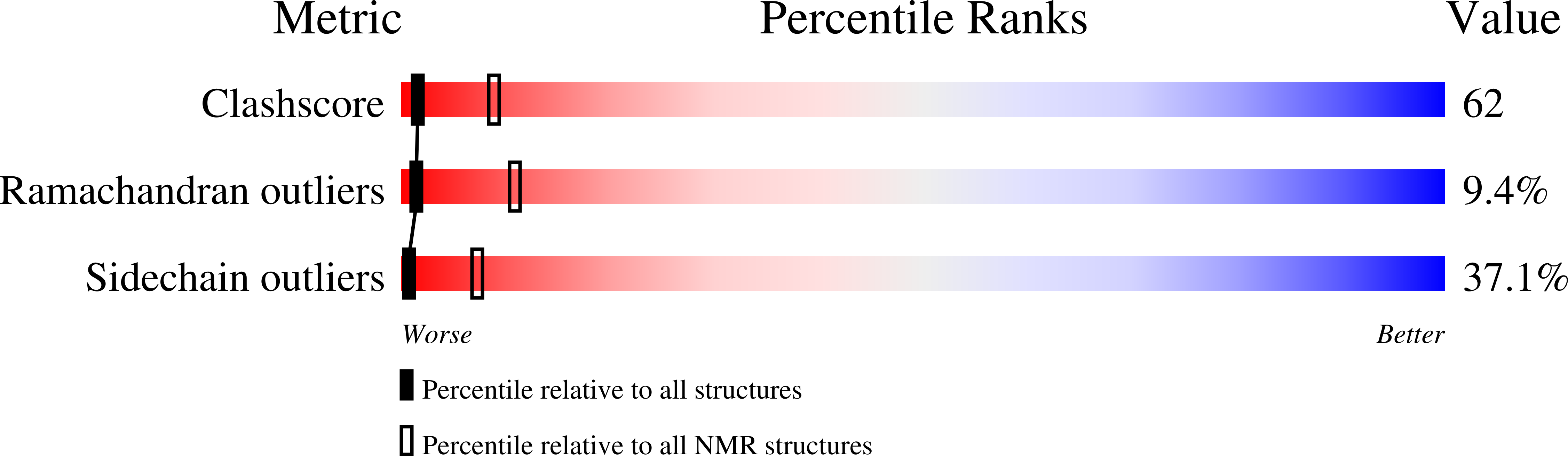Membrane-mediated structural transitions at the cytoplasmic face during integrin activation.
Vinogradova, O., Vaynberg, J., Kong, X., Haas, T.A., Plow, E.F., Qin, J.(2004) Proc Natl Acad Sci U S A 101: 4094-4099
- PubMed: 15024114
- DOI: https://doi.org/10.1073/pnas.0400742101
- Primary Citation of Related Structures:
1S4W, 1S4X - PubMed Abstract:
Cytoplasmic face-mediated integrin inside-out activation remains a paradigm in transmembrane signal transduction. Emerging evidence suggests that this process involves dissociation of the complex between the integrin cytoplasmic tails; however, a dynamic image of how it occurs on the membrane surface remains elusive. We show here that, whereas membrane-proximal helices of integrin alpha/beta cytoplasmic tails associate in cytoplasm-like aqueous medium, they become partially embedded into membrane-mimetic micelles when unclasped. Membrane embedding induces substantial structural changes of the cytoplasmic tails as compared to their aqueous conformations and suggests there may be an upward movement of the membrane-proximal helices into the membrane during their separation. We further demonstrate that the beta3 tail exhibits additional membrane binding site at its C terminus containing the NPLY motif. Talin, a key intracellular integrin activator, recognizes this site as well as the membrane-proximal helix, thereby promoting cytoplasmic tail separation along the membrane surface. These data provide a structural basis of membrane-mediated changes at the cytoplasmic face in regulating integrin activation and signaling.
Organizational Affiliation:
Structural Biology Program, Department of Molecular Cardiology, Joseph J. Jacobs Center for Thrombosis and Vascular Biology, Lerner Research Institute, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195, USA.