The Integration of Recognition and Cleavage: X-Ray Structures of Pre- Transition State and Post-Reactive DNA-Eco RI Endonuclease Complexes
Horvath, M.M., Choi, J., Kim, Y., Wilkosz, P., Rosenberg, J.M.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ENDONUCLEASE ECORI | C [auth A] | 261 | Escherichia coli | Mutation(s): 0 | 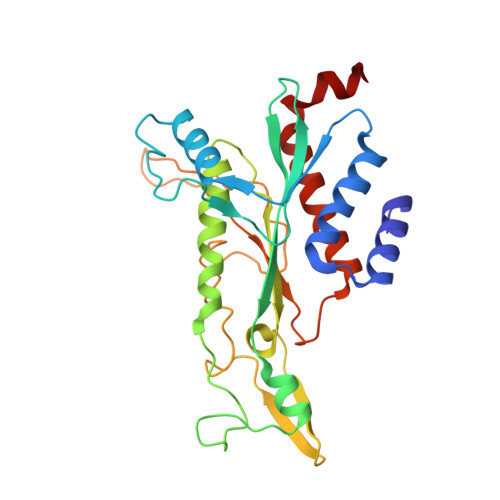 |
UniProt | |||||
Find proteins for P00642 (Escherichia coli) Explore P00642 Go to UniProtKB: P00642 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00642 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar nucleic acids by: Sequence | 3D Structure
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| 5'-D(*TP*CP*GP*CP*GP*)-3' | A [auth M] | 5 | N/A | 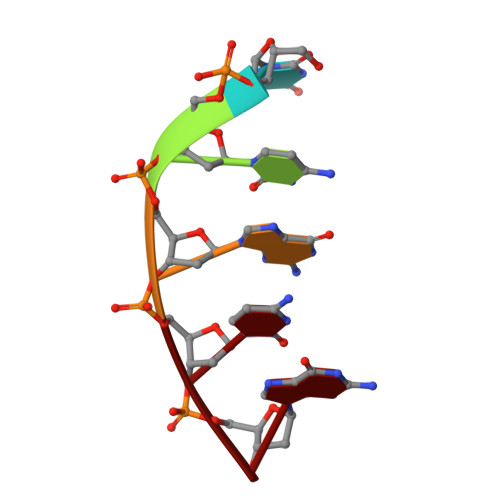 | |
Sequence AnnotationsExpand | |||||
| |||||
Find similar nucleic acids by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| 5'-D(*AP*AP*TP*TP*CP*GP*CP*GP*)-3' | B [auth N] | 8 | N/A | 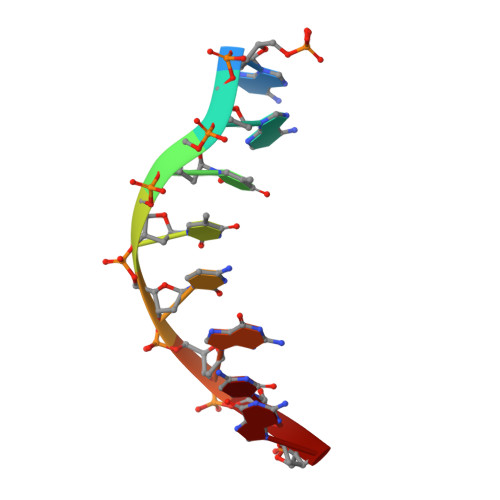 | |
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| MN Query on MN | D [auth A] | MANGANESE (II) ION Mn WAEMQWOKJMHJLA-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 118.4 | α = 90 |
| b = 118.4 | β = 90 |
| c = 49.7 | γ = 120 |
| Software Name | Purpose |
|---|---|
| X-PLOR | model building |
| X-PLOR | refinement |
| X-GEN | data reduction |
| X-GEN | data scaling |
| X-PLOR | phasing |