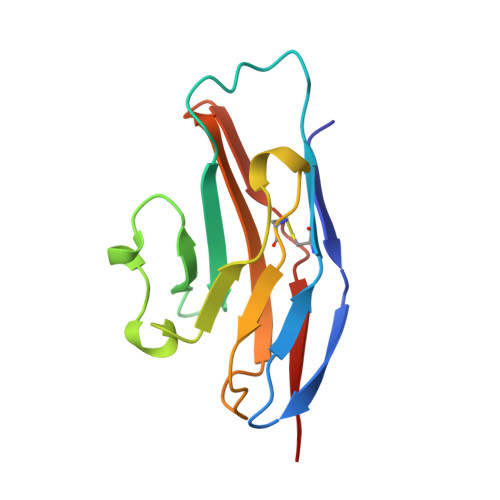
Crystal structure of the N-terminal domain of sialoadhesin in complex with 3' sialyllactose at 1.85 A resolution.
May, A.P., Robinson, R.C., Vinson, M., Crocker, P.R., Jones, E.Y.(1998) Mol Cell 1: 719-728
- PubMed: 9660955
- DOI: https://doi.org/10.1016/s1097-2765(00)80071-4
- Primary Citation of Related Structures:
1QFO, 1QFP - PubMed Abstract:
The structure of the functional N-terminal domain from the extracellular region of the cell surface receptor sialoadhesin has been determined in complex with the oligosaccharide 3' sialyllactose. This provides structural information for the siglec family of proteins. The structure conforms to the V-set immunoglobulin-like fold but contains several distinctive features, including an intra-beta sheet disulphide and a splitting of the standard beta strand G into two shorter strands. These novel features appear important in adapting the V-set fold for sialic acid-mediated recognition. Analysis of the complex with 3'sialyllactose highlights three residues, conserved throughout the siglec family, as key features of the sialic acid-binding template. The complex is representative of the functional recognition interaction with carbohydrate and as such provides detailed information for a heterotypic cell adhesion interaction.
Organizational Affiliation:
Laboratory of Molecular Biophysics, University of Oxford, United Kingdom.