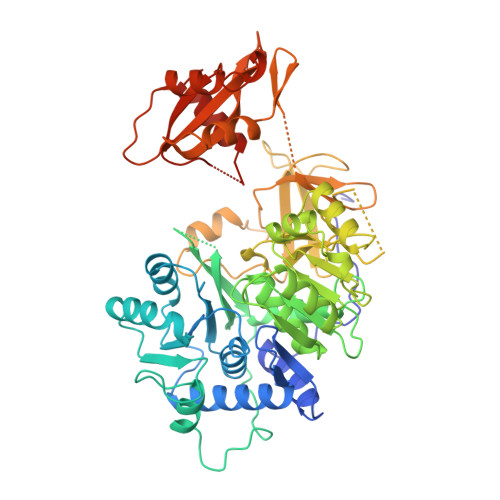
Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes.
Conti, E., Franks, N.P., Brick, P.(1996) Structure 4: 287-298
- PubMed: 8805533
- DOI: https://doi.org/10.1016/s0969-2126(96)00033-0
- Primary Citation of Related Structures:
1LCI - PubMed Abstract:
Firefly luciferase is a 62 kDa protein that catalyzes the production of light. In the presence of MgATP and molecular oxygen, the enzyme oxidizes its substrate, firefly luciferin, emitting yellow-green light. The reaction proceeds through activation of the substrate to form an adenylate intermediate. Firefly luciferase shows extensive sequence homology with a number of enzymes that utilize ATP in adenylation reactions.
Organizational Affiliation:
Biophysics Section, Blackett Laboratory, Imperial College, London SW7 2BZ, UK.