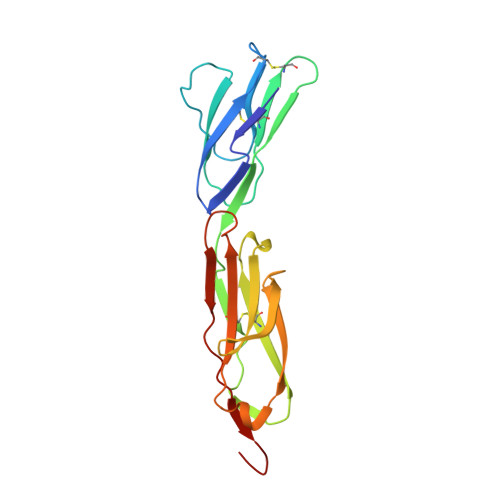
A dimeric crystal structure for the N-terminal two domains of intercellular adhesion molecule-1.
Casasnovas, J.M., Stehle, T., Liu, J.H., Wang, J.H., Springer, T.A.(1998) Proc Natl Acad Sci U S A 95: 4134-4139
- PubMed: 9539702
- DOI: https://doi.org/10.1073/pnas.95.8.4134
- Primary Citation of Related Structures:
1IC1 - PubMed Abstract:
The 3.0-A structure of a 190-residue fragment of intercellular adhesion molecule-1 (ICAM-1, CD54) reveals two tandem Ig-superfamily (IgSF) domains. Each of two independent molecules dimerizes identically with a symmetry-related molecule over a hydrophobic interface on the BED sheet of domain 1, in agreement with dimerization of ICAM-1 on the cell surface. The residues that bind to the integrin LFA-1 are well oriented for bivalent binding in the dimer, with the critical Glu-34 residues pointing away from each other on the periphery. Residues that bind to rhinovirus are in the flexible BC and FG loops at the tip of domain 1, and these and the upper half of domain 1 are well exposed in the dimer for docking to virus. By contrast, a residue important for binding to Plasmodium falciparum-infected erythrocytes is in the dimer interface. The presence of A' strands in both domains 1 and 2, conserved hydrogen bonds at domain junctions, and elaborate hydrogen bond networks around the key integrin binding residues in domain 1 make these domains suited to resist tensile forces during adhesive interactions. A subdivision of the intermediate (I) set of IgSF domains is proposed in which domain 1 of ICAM-1 and previously described I set domains belong to the I1 set and domain 2 of ICAM-1, ICAM-2, and vascular cell adhesion molecule-1 belong to the I2 set.
Organizational Affiliation:
The Center for Blood Research and Department of Pathology, Harvard Medical School, 200 Longwood Avenue, Boston, MA 02115, USA.