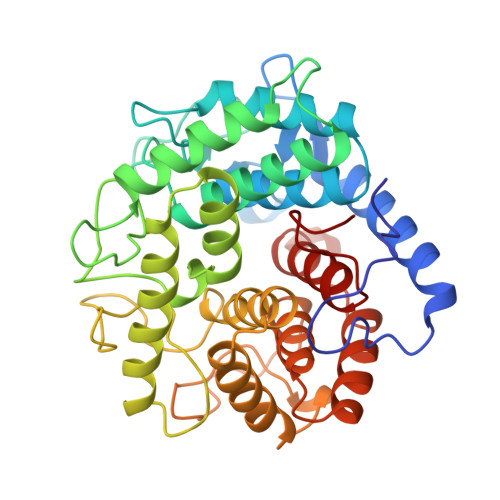
The crystal structure of endoglucanase CelA, a family 8 glycosyl hydrolase from Clostridium thermocellum.
Alzari, P.M., Souchon, H., Dominguez, R.(1996) Structure 4: 265-275
- PubMed: 8805535
- DOI: https://doi.org/10.1016/s0969-2126(96)00031-7
- Primary Citation of Related Structures:
1CEM - PubMed Abstract:
Cellulases, which catalyze the hydrolysis of glycosidic bonds in cellulose, can be classified into several different protein families. Endoglucanase CelA is a member of glycosyl hydrolase family 8, a family for which no structural information was previously available.
Organizational Affiliation:
Unité d'lmmunologie Structurale and URA 1961 CNRS, Institut Pasteur, Paris, France.