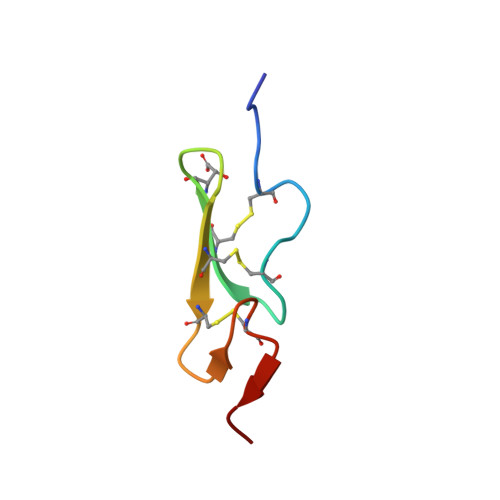
How an epidermal growth factor (EGF)-like domain binds calcium. High resolution NMR structure of the calcium form of the NH2-terminal EGF-like domain in coagulation factor X.
Selander-Sunnerhagen, M., Ullner, M., Persson, E., Teleman, O., Stenflo, J., Drakenberg, T.(1992) J Biol Chem 267: 19642-19649
- PubMed: 1527084
- DOI: https://doi.org/10.2210/pdb1ccf/pdb
- Primary Citation of Related Structures:
1CCF - PubMed Abstract:
Domains homologous to the epidermal growth factor (EGF) are important building blocks for extracellular proteins. Proteins containing these domains have been shown to function in such diverse biological processes as blood coagulation, complement activation, and the developmental determination of embryonic cell fates. Many of these proteins require calcium for their biological function. In the case of coagulation factors IX and X and anticoagulants proteins C and S, calcium has been found to bind to the EGF-like domains. We have now determined the three-dimensional structure of the calcium-bound form of the NH2-terminal EGF-like domain in coagulation factor X by two-dimensional NMR and simulated folding. Ligands to the calcium ion are the two backbone carbonyls in Gly-47 and Gly-64, as well as the side chains in Gln-49, erythro-beta-hydroxyaspartic acid (Hya) 63, and possibly Asp-46. The conserved Asp-48 is not a ligand in our present structures. The remaining ligands are assumed to be solvent molecules or, in the intact protein, ligands from neighboring domains. Other proteins interacting in a calcium-dependent manner may also contribute ligands. A comparison with the calcium-free form shows that calcium binding induces strictly local structural changes in the domain. Residues corresponding to the side chain ligands in factor X are conserved in many other proteins, such as the integral membrane protein TAN-1 of human lymphocytes and its developmentally important homolog, Notch, in Drosophila. Calcium binding to EGF-like domains may be crucial for numerous protein-protein interactions involving EGF-like domains in coagulation factors, plasma proteins, and membrane proteins. Therefore, there is reason to believe that this novel calcium site plays an important role in the biochemistry of extracellular proteins.
Organizational Affiliation:
Department of Physical Chemistry 2, University of Lund, Sweden.