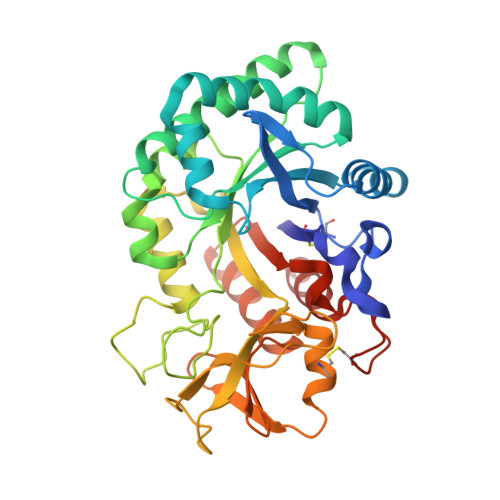
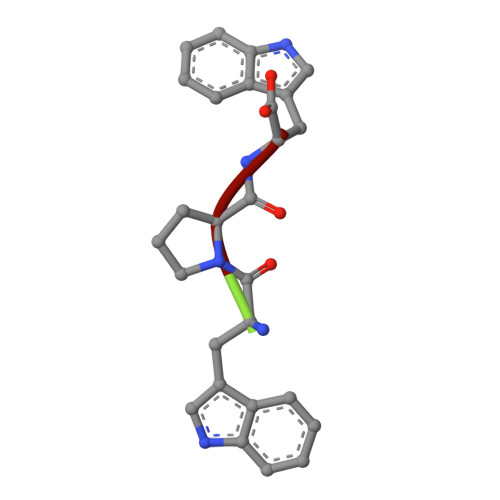
Recognition of peptide ligands by signalling protein from porcine mammary gland (SPP-40): Crystal structure of the complex of SPP-40 with a peptide Trp-Pro-Trp at 2.45A resolution
Srivastava, D.B., Ethayathulla, A.S., Kumar, J., Singh, N., Somvanshi, R.K., Sharma, S., Dey, S., Kaur, P., Singh, T.P.To be published.