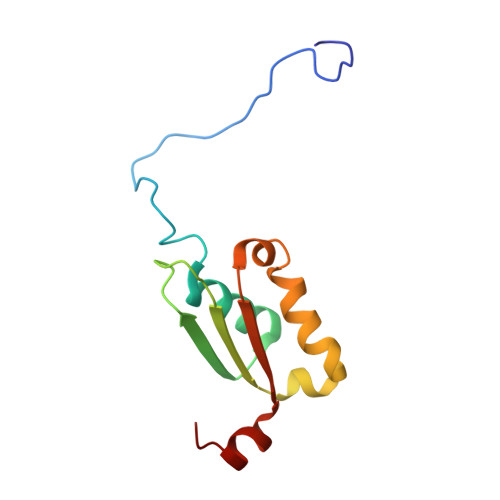
Solution structure of a BolA-like protein from Mus musculus
Kasai, T., Inoue, M., Koshiba, S., Yabuki, T., Aoki, M., Nunokawa, E., Seki, E., Matsuda, T., Matsuda, N., Tomo, Y., Shirouzu, M., Terada, T., Obayashi, N., Hamana, H., Shinya, N., Tatsuguchi, A., Yasuda, S., Yoshida, M., Hirota, H., Matsuo, Y., Tani, K., Suzuki, H., Arakawa, T., Carninci, P., Kawai, J., Hayashizaki, Y., Kigawa, T., Yokoyama, S.(2004) Protein Sci 13: 545-548
- PubMed: 14718656
- DOI: https://doi.org/10.1110/ps.03401004
- Primary Citation of Related Structures:
1V9J - PubMed Abstract:
The BolA-like proteins are widely conserved from prokaryotes to eukaryotes. The BolA-like proteins seem to be involved in cell proliferation or cell-cycle regulation, but the molecular function is still unknown. Here we determined the structure of a mouse BolA-like protein. The overall topology is alphabetabetaalphaalphabetaalpha, in which beta(1) and beta(2) are antiparallel, and beta(3) is parallel to beta(2). This fold is similar to the class II KH fold, except for the absence of the GXXG loop, which is well conserved in the KH fold. The conserved residues in the BolA-like proteins are assembled on the one side of the protein.
Organizational Affiliation:
Protein Research Group, RIKEN Genomic Sciences Center, 1-7-22 Suehiro-cho, Tsurumi, Yokohama 230-0045, Japan.